In vivo evaluation of candidate allele-specific mutant huntingtin gene silencing antisense oligonucleotides
- PMID: 25101598
- PMCID: PMC4429695
- DOI: 10.1038/mt.2014.153
In vivo evaluation of candidate allele-specific mutant huntingtin gene silencing antisense oligonucleotides
Abstract
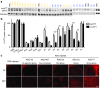
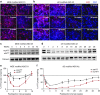
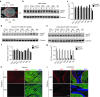
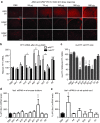
Huntington disease (HD) is a dominant, genetic neurodegenerative disease characterized by progressive loss of voluntary motor control, psychiatric disturbance, and cognitive decline, for which there is currently no disease-modifying therapy. HD is caused by the expansion of a CAG tract in the huntingtin (HTT) gene. The mutant HTT protein (muHTT) acquires toxic functions, and there is significant evidence that muHTT lowering would be therapeutically efficacious. However, the wild-type HTT protein (wtHTT) serves vital functions, making allele-specific muHTT lowering strategies potentially safer than nonselective strategies. CAG tract expansion is associated with single nucleotide polymorphisms (SNPs) that can be targeted by gene silencing reagents such as antisense oligonucleotides (ASOs) to accomplish allele-specific muHTT lowering. Here we evaluate ASOs targeted to HD-associated SNPs in acute in vivo studies including screening, distribution, duration of action and dosing, using a humanized mouse model of HD, Hu97/18, that is heterozygous for the targeted SNPs. We have identified four well-tolerated lead ASOs that potently and selectively silence muHTT at a broad range of doses throughout the central nervous system for 16 weeks or more after a single intracerebroventricular (ICV) injection. With further validation, these ASOs could provide a therapeutic option for individuals afflicted with HD.
Figures




References
-
- Ross CA, Tabrizi SJ. Huntington's disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10:83–98. - PubMed
-
- The Huntington's Disease Collaborative Research Group A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72:971–983. - PubMed
-
- Zuccato C, Valenza M, Cattaneo E. Molecular mechanisms and potential therapeutical targets in Huntington's disease. Physiol Rev. 2010;90:905–981. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

