Arabidopsis miR171-targeted scarecrow-like proteins bind to GT cis-elements and mediate gibberellin-regulated chlorophyll biosynthesis under light conditions
- PMID: 25101599
- PMCID: PMC4125095
- DOI: 10.1371/journal.pgen.1004519
Arabidopsis miR171-targeted scarecrow-like proteins bind to GT cis-elements and mediate gibberellin-regulated chlorophyll biosynthesis under light conditions
Abstract
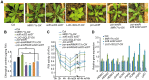
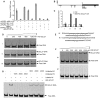
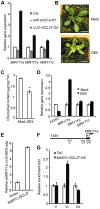
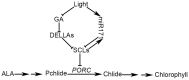
An extraordinarily precise regulation of chlorophyll biosynthesis is essential for plant growth and development. However, our knowledge on the complex regulatory mechanisms of chlorophyll biosynthesis is very limited. Previous studies have demonstrated that miR171-targeted scarecrow-like proteins (SCL6/22/27) negatively regulate chlorophyll biosynthesis via an unknown mechanism. Here we showed that SCLs inhibit the expression of the key gene encoding protochlorophyllide oxidoreductase (POR) in light-grown plants, but have no significant effect on protochlorophyllide biosynthesis in etiolated seedlings. Histochemical analysis of β-glucuronidase (GUS) activity in transgenic plants expressing pSCL27::rSCL27-GUS revealed that SCL27-GUS accumulates at high levels and suppresses chlorophyll biosynthesis at the leaf basal proliferation region during leaf development. Transient gene expression assays showed that the promoter activity of PORC is indeed regulated by SCL27. Consistently, chromatin immunoprecipitation and quantitative PCR assays showed that SCL27 binds to the promoter region of PORC in vivo. An electrophoretic mobility shift assay revealed that SCL27 is directly interacted with G(A/G)(A/T)AA(A/T)GT cis-elements of the PORC promoter. Furthermore, genetic analysis showed that gibberellin (GA)-regulated chlorophyll biosynthesis is mediated, at least in part, by SCLs. We demonstrated that SCL27 interacts with DELLA proteins in vitro and in vivo by yeast-two-hybrid and coimmunoprecipitation analysis and found that their interaction reduces the binding activity of SCL27 to the PORC promoter. Additionally, we showed that SCL27 activates MIR171 gene expression, forming a feedback regulatory loop. Taken together, our data suggest that the miR171-SCL module is critical for mediating GA-DELLA signaling in the coordinate regulation of chlorophyll biosynthesis and leaf growth in light.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
The SWI2/SNF2 Chromatin-Remodeling ATPase BRAHMA Regulates Chlorophyll Biosynthesis in Arabidopsis.Mol Plant. 2017 Jan 9;10(1):155-167. doi: 10.1016/j.molp.2016.11.003. Epub 2016 Nov 16. Mol Plant. 2017. PMID: 27865928
-
DELLAs regulate chlorophyll and carotenoid biosynthesis to prevent photooxidative damage during seedling deetiolation in Arabidopsis.Plant Cell. 2011 May;23(5):1849-60. doi: 10.1105/tpc.111.085233. Epub 2011 May 13. Plant Cell. 2011. PMID: 21571951 Free PMC article.
-
GNC and CGA1 modulate chlorophyll biosynthesis and glutamate synthase (GLU1/Fd-GOGAT) expression in Arabidopsis.PLoS One. 2011;6(11):e26765. doi: 10.1371/journal.pone.0026765. Epub 2011 Nov 10. PLoS One. 2011. PMID: 22102866 Free PMC article.
-
REVEILLE1 promotes NADPH: protochlorophyllide oxidoreductase A expression and seedling greening in Arabidopsis.Photosynth Res. 2015 Dec;126(2-3):331-40. doi: 10.1007/s11120-015-0146-5. Epub 2015 Apr 25. Photosynth Res. 2015. PMID: 25910753
-
An Arabidopsis porB porC double mutant lacking light-dependent NADPH:protochlorophyllide oxidoreductases B and C is highly chlorophyll-deficient and developmentally arrested.Plant J. 2003 Jul;35(2):141-53. doi: 10.1046/j.1365-313x.2003.01798.x. Plant J. 2003. PMID: 12848821
Cited by
-
Network of GRAS transcription factors in plant development, fruit ripening and stress responses.Hortic Res. 2023 Sep 27;10(12):uhad220. doi: 10.1093/hr/uhad220. eCollection 2023 Dec. Hortic Res. 2023. PMID: 38077496 Free PMC article.
-
Identification of miRNAs and their targets through high-throughput sequencing and degradome analysis in male and female Asparagus officinalis.BMC Plant Biol. 2016 Apr 12;16:80. doi: 10.1186/s12870-016-0770-z. BMC Plant Biol. 2016. PMID: 27068118 Free PMC article.
-
Integrated analysis of the transcriptome, sRNAome, and degradome reveals the network regulating fruit skin coloration in sponge gourd (Luffa cylindrica).Sci Rep. 2022 Feb 28;12(1):3338. doi: 10.1038/s41598-022-07431-w. Sci Rep. 2022. PMID: 35228643 Free PMC article.
-
Multifarious and Interactive Roles of GRAS Transcription Factors During Arbuscular Mycorrhiza Development.Front Plant Sci. 2022 Mar 28;13:836213. doi: 10.3389/fpls.2022.836213. eCollection 2022. Front Plant Sci. 2022. PMID: 35419017 Free PMC article. Review.
-
Unravelling miRNA regulation in yield of rice (Oryza sativa) based on differential network model.Sci Rep. 2018 May 31;8(1):8498. doi: 10.1038/s41598-018-26438-w. Sci Rep. 2018. PMID: 29855560 Free PMC article.
References
-
- Goslings D, Meskauskiene R, Kim C, Lee KP, Nater M, et al. (2004) Concurrent interactions of heme and FLU with Glu tRNA reductase (HEMA1), the target of metabolic feedback inhibition of tetrapyrrole biosynthesis, in dark- and light-grown Arabidopsis plants. Plant J 40: 957–967. - PubMed
-
- Krieger-Liszkay A (2005) Singlet oxygen production in photosynthesis. J Exp Bot 56: 337–346. - PubMed
-
- Reinbothe C, El Bakkouri M, Buhr F, Muraki N, Nomata J, et al. (2010) Chlorophyll biosynthesis: spotlight on protochlorophyllide reduction. Trends Plant Sci 15: 614–624. - PubMed
-
- Mochizuki N, Tanaka R, Grimm B, Masuda T, Moulin M, et al. (2010) The cell biology of tetrapyrroles: a life and death struggle. Trends Plant Sci 15: 488–498. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

