Transcription addiction: can we garner the Yin and Yang functions of E2F1 for cancer therapy?
- PMID: 25101673
- PMCID: PMC4454301
- DOI: 10.1038/cddis.2014.326
Transcription addiction: can we garner the Yin and Yang functions of E2F1 for cancer therapy?
Abstract
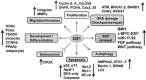
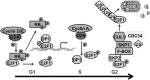
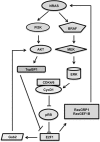
Classically, as a transcription factor family, the E2Fs are known to regulate the expression of various genes whose products are involved in a multitude of biological functions, many of which are deregulated in diseases including cancers. E2F is deregulated and hyperactive in most human cancers with context dependent, dichotomous and contradictory roles in almost all cancers. Cancer cells have an insatiable demand for transcription to ensure that gene products are available to sustain various biological processes that support their rapid growth and survival. In this context, cutting-off hyperactivity of transcription factors that support transcription dependence could be a valuable therapeutic strategy. However, one of the greatest challenges of targeting a transcription factor is the global effects on non-cancerous cells given that they control cellular functions in general. Recently, there is growing realization regarding the possibility to target the oncogenic activation of transcription factors to modulate transcription addiction without affecting the normal activity required for cell functions. In this review, we used E2F1 as a prototype transcription factor to address transcription factor activity in cancer cell functions. We focused on melanoma considering that E2F1 executes critical functions in response to UV, an etiological factor of cutaneous melanoma and lies immediately downstream of the CDKN2A/pRb axis, which is frequently deregulated in melanoma. Further, activation of E2F1 in melanomas can also occur independent of loss of CDKN2A. Given its activated status and the ability to transcriptionally control a plethora of genes involved in regulating melanoma development and progression, we review the current literature on its differential role in controlling signaling pathways involved in melanoma as well as therapeutic resistance, and discuss the practical value of weaning melanoma cells from E2F1-mediated transcription dependence for melanoma management.
Figures

Similar articles
-
Activation of a cAMP pathway and induction of melanogenesis correlate with association of p16(INK4) and p27(KIP1) to CDKs, loss of E2F-binding activity, and premature senescence of human melanocytes.Exp Cell Res. 1999 Dec 15;253(2):561-72. doi: 10.1006/excr.1999.4688. Exp Cell Res. 1999. PMID: 10585280
-
MiR-15 and miR-16 are direct transcriptional targets of E2F1 that limit E2F-induced proliferation by targeting cyclin E.Mol Cancer Res. 2011 Apr;9(4):440-7. doi: 10.1158/1541-7786.MCR-10-0344. Epub 2011 Mar 31. Mol Cancer Res. 2011. PMID: 21454377
-
Cyclin E and c-Myc promote cell proliferation in the presence of p16INK4a and of hypophosphorylated retinoblastoma family proteins.EMBO J. 1997 Sep 1;16(17):5322-33. doi: 10.1093/emboj/16.17.5322. EMBO J. 1997. PMID: 9311992 Free PMC article.
-
[Molecular mechanisms controlling the cell cycle: fundamental aspects and implications for oncology].Cancer Radiother. 2001 Apr;5(2):109-29. doi: 10.1016/s1278-3218(01)00087-7. Cancer Radiother. 2001. PMID: 11355576 Review. French.
-
E2F - at the crossroads of life and death.Trends Cell Biol. 2008 Nov;18(11):528-35. doi: 10.1016/j.tcb.2008.08.003. Epub 2008 Sep 18. Trends Cell Biol. 2008. PMID: 18805009 Review.
Cited by
-
Chemotherapy Resistance in Advanced Ovarian Cancer Patients.Biomark Cancer. 2019 Jul 5;11:1179299X19860815. doi: 10.1177/1179299X19860815. eCollection 2019. Biomark Cancer. 2019. PMID: 31308780 Free PMC article. Review.
-
Inhibition of PKC/MEK pathway suppresses β1-integrin and mitigates breast cancer cells proliferation.Toxicol Rep. 2021 Jul 21;8:1530-1537. doi: 10.1016/j.toxrep.2021.07.012. eCollection 2021. Toxicol Rep. 2021. PMID: 34408972 Free PMC article.
-
Exploring the genetic profiles linked to senescence in thyroid tumors: insights on predicting disease progression and immune responses.Front Oncol. 2025 Feb 6;15:1545656. doi: 10.3389/fonc.2025.1545656. eCollection 2025. Front Oncol. 2025. PMID: 39980566 Free PMC article.
-
Executioner Caspase-3 and 7 Deficiency Reduces Myocyte Number in the Developing Mouse Heart.PLoS One. 2015 Jun 29;10(6):e0131411. doi: 10.1371/journal.pone.0131411. eCollection 2015. PLoS One. 2015. PMID: 26121671 Free PMC article.
-
The hnRNP RALY regulates transcription and cell proliferation by modulating the expression of specific factors including the proliferation marker E2F1.J Biol Chem. 2017 Dec 1;292(48):19674-19692. doi: 10.1074/jbc.M117.795591. Epub 2017 Sep 27. J Biol Chem. 2017. PMID: 28972179 Free PMC article.
References
-
- Kovesdi I, Reichel R, Nevins JR. Identification of a cellular transcription factor involved in E1A trans-activation. Cell. 1986;45:219–228. - PubMed
-
- Polager S, Ginsberg D. E2F—at the crossroads of life and death. Trends Cell Biol. 2008;18:528–535. - PubMed
-
- Mundle SD, Saberwal G. Evolving intricacies and implications of E2F1 regulation. FASEB J. 2003;17:569–574. - PubMed
-
- Bracken AP, Ciro M, Cocito A, Helin K. E2F target genes: unraveling the biology. Trends Biochem Sci. 2004;29:409–417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

