A novel androstenedione derivative induces ROS-mediated autophagy and attenuates drug resistance in osteosarcoma by inhibiting macrophage migration inhibitory factor (MIF)
- PMID: 25101674
- PMCID: PMC4454296
- DOI: 10.1038/cddis.2014.300
A novel androstenedione derivative induces ROS-mediated autophagy and attenuates drug resistance in osteosarcoma by inhibiting macrophage migration inhibitory factor (MIF)
Abstract
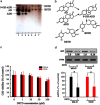
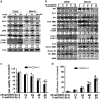
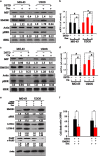
Osteosarcoma is a common primary bone tumor in children and adolescents. The drug resistance of osteosarcoma leads to high lethality. Macrophage migration inhibitory factor (MIF) is an inflammation-related cytokine implicated in the chemoresistance of breast cancer. In this study, we isolated a novel androstenedione derivative identified as 3,4-dihydroxy-9,10-secoandrosta-1,3,5,7-tetraene-9,17-dione (DSTD). DSTD could inhibit MIF expression in MG-63 and U2OS cells. The inhibition of MIF by DSTD promoted autophagy by inducing Bcl-2 downregulation and the translocation of HMGB1. N-acetyl-L-cysteine (NAC) and 3-methyladenine (3-MA) attenuated DSTD-induced autophagy but promoted cell death, suggesting that DSTD induced ROS-mediated autophagy to rescue cell death. However, in the presence of chemotherapy drugs, DSTD enhanced the chemosensitivity by decreasing the HMGB1 level. Our data suggest MIF inhibition as a therapeutic strategy for overcoming drug resistance in osteosarcoma.
Figures




Similar articles
-
Macrophage migration inhibitory factor promotes osteosarcoma growth and lung metastasis through activating the RAS/MAPK pathway.Cancer Lett. 2017 Sep 10;403:271-279. doi: 10.1016/j.canlet.2017.06.011. Epub 2017 Jun 19. Cancer Lett. 2017. PMID: 28642171
-
Macrophage migration inhibitory factor induces autophagy via reactive oxygen species generation.PLoS One. 2012;7(5):e37613. doi: 10.1371/journal.pone.0037613. Epub 2012 May 22. PLoS One. 2012. PMID: 22629429 Free PMC article.
-
Aloe-emodin-mediated photodynamic therapy induces autophagy and apoptosis in human osteosarcoma cell line MG‑63 through the ROS/JNK signaling pathway.Oncol Rep. 2016 Jun;35(6):3209-15. doi: 10.3892/or.2016.4703. Epub 2016 Mar 24. Oncol Rep. 2016. PMID: 27035222 Free PMC article.
-
Autophagy in Osteosarcoma.Adv Exp Med Biol. 2020;1258:167-175. doi: 10.1007/978-3-030-43085-6_11. Adv Exp Med Biol. 2020. PMID: 32767241 Free PMC article. Review.
-
Role of autophagy in drug resistance and regulation of osteosarcoma (Review).Mol Clin Oncol. 2022 Mar;16(3):72. doi: 10.3892/mco.2022.2505. Epub 2022 Feb 1. Mol Clin Oncol. 2022. PMID: 35251623 Free PMC article. Review.
Cited by
-
Quercetin blocks t-AUCB-induced autophagy by Hsp27 and Atg7 inhibition in glioblastoma cells in vitro.J Neurooncol. 2016 Aug;129(1):39-45. doi: 10.1007/s11060-016-2149-2. Epub 2016 May 12. J Neurooncol. 2016. PMID: 27174198
-
Role and mechanism of action of LAPTM4B in EGFR-mediated autophagy.Oncol Lett. 2022 Apr;23(4):109. doi: 10.3892/ol.2022.13229. Epub 2022 Feb 7. Oncol Lett. 2022. PMID: 35242237 Free PMC article. Review.
-
The Antitumor Efficacy of β-Elemene by Changing Tumor Inflammatory Environment and Tumor Microenvironment.Biomed Res Int. 2020 Feb 19;2020:6892961. doi: 10.1155/2020/6892961. eCollection 2020. Biomed Res Int. 2020. PMID: 32149121 Free PMC article. Review.
-
p73 regulates basal and starvation-induced liver metabolism in vivo.Oncotarget. 2015 Oct 20;6(32):33178-90. doi: 10.18632/oncotarget.5090. Oncotarget. 2015. PMID: 26375672 Free PMC article.
-
The progression of HMGB1-induced autophagy in cancer biology.Onco Targets Ther. 2018 Dec 31;12:365-377. doi: 10.2147/OTT.S185876. eCollection 2019. Onco Targets Ther. 2018. PMID: 30643434 Free PMC article. Review.
References
-
- Park JY, Kim YW, Park YK. Nrf2 expression is associated with poor outcome in osteosarcoma. Pathology. 2012;44:617–621. - PubMed
-
- Moriceau G, Roelofs AJ, Brion R, Redini F, Ebetion FH, Rogers MJ, et al. Synergistic inhibitory effect of apomine and lovastatin on osteosarcoma cell growth. Cancer. 2012;118:750–760. - PubMed
-
- Rosen G, Murphy ML, Huvos AG, Gutierrez M, Marcove RC. Chemotherapy, en bloc resection, and prosthetic bone replacement in the treatment of osteogenic sarcoma. Cancer. 1976;37:1–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

