Unique nucleotide sequence-guided assembly of repetitive DNA parts for synthetic biology applications
- PMID: 25101822
- PMCID: PMC4899833
- DOI: 10.1038/nprot.2014.145
Unique nucleotide sequence-guided assembly of repetitive DNA parts for synthetic biology applications
Abstract
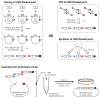
Recombination-based DNA construction methods, such as Gibson assembly, have made it possible to easily and simultaneously assemble multiple DNA parts, and they hold promise for the development and optimization of metabolic pathways and functional genetic circuits. Over time, however, these pathways and circuits have become more complex, and the increasing need for standardization and insulation of genetic parts has resulted in sequence redundancies--for example, repeated terminator and insulator sequences--that complicate recombination-based assembly. We and others have recently developed DNA assembly methods, which we refer to collectively as unique nucleotide sequence (UNS)-guided assembly, in which individual DNA parts are flanked with UNSs to facilitate the ordered, recombination-based assembly of repetitive sequences. Here we present a detailed protocol for UNS-guided assembly that enables researchers to convert multiple DNA parts into sequenced, correctly assembled constructs, or into high-quality combinatorial libraries in only 2-3 d. If the DNA parts must be generated from scratch, an additional 2-5 d are necessary. This protocol requires no specialized equipment and can easily be implemented by a student with experience in basic cloning techniques.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures



Similar articles
-
Rapid construction of insulated genetic circuits via synthetic sequence-guided isothermal assembly.Nucleic Acids Res. 2014 Jan;42(1):681-9. doi: 10.1093/nar/gkt860. Epub 2013 Sep 26. Nucleic Acids Res. 2014. PMID: 24078086 Free PMC article.
-
Assembly of Multigene Constructs Using the Modular Cloning System MoClo.Methods Mol Biol. 2020;2205:125-141. doi: 10.1007/978-1-0716-0908-8_8. Methods Mol Biol. 2020. PMID: 32809197
-
A Modular Approach to Building Complex Synthetic Circuits.Methods Mol Biol. 2017;1651:231-248. doi: 10.1007/978-1-4939-7223-4_17. Methods Mol Biol. 2017. PMID: 28801911
-
Bricks and blueprints: methods and standards for DNA assembly.Nat Rev Mol Cell Biol. 2015 Sep;16(9):568-76. doi: 10.1038/nrm4014. Epub 2015 Jun 17. Nat Rev Mol Cell Biol. 2015. PMID: 26081612 Review.
-
[Perspective on the novel methods for DNA assembly].Sheng Wu Gong Cheng Xue Bao. 2013 Aug;29(8):1113-22. Sheng Wu Gong Cheng Xue Bao. 2013. PMID: 24364348 Review. Chinese.
Cited by
-
Metabolically engineered recombinant Saccharomyces cerevisiae for the production of 2-Deoxy-scyllo-inosose (2-DOI).Metab Eng Commun. 2020 May 31;11:e00134. doi: 10.1016/j.mec.2020.e00134. eCollection 2020 Dec. Metab Eng Commun. 2020. PMID: 32670790 Free PMC article.
-
Dipeptidyl peptidase 9 triggers BRCA2 degradation and promotes DNA damage repair.EMBO Rep. 2022 Oct 6;23(10):e54136. doi: 10.15252/embr.202154136. Epub 2022 Aug 1. EMBO Rep. 2022. PMID: 35912982 Free PMC article.
-
Generation of a Collection of Mutant Tomato Lines Using Pooled CRISPR Libraries.Plant Physiol. 2017 Aug;174(4):2023-2037. doi: 10.1104/pp.17.00489. Epub 2017 Jun 23. Plant Physiol. 2017. PMID: 28646085 Free PMC article.
-
Synthetic biology and artificial intelligence in crop improvement.Plant Commun. 2025 Feb 10;6(2):101220. doi: 10.1016/j.xplc.2024.101220. Epub 2024 Dec 12. Plant Commun. 2025. PMID: 39668563 Free PMC article. Review.
-
The histone chaperone HIR maintains chromatin states to control nitrogen assimilation and fungal virulence.Cell Rep. 2021 Jul 20;36(3):109406. doi: 10.1016/j.celrep.2021.109406. Cell Rep. 2021. PMID: 34289370 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

