Toward understanding the functional role of Ss-RIOK-1, a RIO protein kinase-encoding gene of Strongyloides stercoralis
- PMID: 25101874
- PMCID: PMC4125297
- DOI: 10.1371/journal.pntd.0003062
Toward understanding the functional role of Ss-RIOK-1, a RIO protein kinase-encoding gene of Strongyloides stercoralis
Abstract
Background: Some studies of Saccharomyces cerevisiae and mammals have shown that RIO protein kinases (RIOKs) are involved in ribosome biogenesis, cell cycle progression and development. However, there is a paucity of information on their functions in parasitic nematodes. We aimed to investigate the function of RIOK-1 encoding gene from Strongyloides stercoralis, a nematode parasitizing humans and dogs.
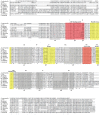
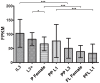
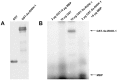
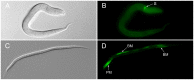
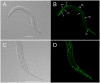
Methodology/principal findings: The RIOK-1 protein-encoding gene Ss-riok-1 was characterized from S. stercoralis. The full-length cDNA, gDNA and putative promoter region of Ss-riok-1 were isolated and sequenced. The cDNA comprises 1,828 bp, including a 377 bp 5'-UTR, a 17 bp 3'-UTR and a 1,434 bp ORF encoding a protein of 477 amino acids containing a RIOK-1 signature motif. The genomic sequence of the Ss-riok-1 coding region is 1,636 bp in length and has three exons and two introns. The putative promoter region comprises 4,280 bp and contains conserved promoter elements, including four CAAT boxes, 12 GATA boxes, eight E-boxes (CANNTG) and 38 TATA boxes. The Ss-riok-1 gene is transcribed throughout all developmental stages with the highest transcript abundance in the infective third-stage larva (iL3). Recombinant Ss-RIOK-1 is an active kinase, capable of both phosphorylation and auto-phosphorylation. Patterns of transcriptional reporter expression in transgenic S. stercoralis larvae indicated that Ss-RIOK-1 is expressed in neurons of the head, body and tail as well as in pharynx and hypodermis.
Conclusions/significance: The characterization of the molecular and the temporal and spatial expression patterns of the encoding gene provide first clues as to functions of RIOKs in the biological processes of parasitic nematodes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Functional genomic exploration reveals that Ss-RIOK-1 is essential for the development and survival of Strongyloides stercoralis larvae.Int J Parasitol. 2017 Dec;47(14):933-940. doi: 10.1016/j.ijpara.2017.06.005. Epub 2017 Aug 3. Int J Parasitol. 2017. PMID: 28780152 Free PMC article.
-
Exploring features and function of Ss-riok-3, an enigmatic kinase gene from Strongyloides stercoralis.Parasit Vectors. 2014 Dec 5;7:561. doi: 10.1186/s13071-014-0561-z. Parasit Vectors. 2014. PMID: 25477034 Free PMC article.
-
Structural and developmental expression of Ss-riok-2, an RIO protein kinase encoding gene of Strongyloides stercoralis.Sci Rep. 2017 Aug 18;7(1):8693. doi: 10.1038/s41598-017-07991-2. Sci Rep. 2017. PMID: 28821723 Free PMC article.
-
Assessment of the global paradigms of genetic variability in Strongyloides stercoralis infrapopulations determined by mitochondrial DNA sequences.Comp Immunol Microbiol Infect Dis. 2019 Dec;67:101354. doi: 10.1016/j.cimid.2019.101354. Epub 2019 Sep 26. Comp Immunol Microbiol Infect Dis. 2019. PMID: 31586852 Review.
-
Chemo- and thermosensory neurons: structure and function in animal parasitic nematodes.Vet Parasitol. 1999 Aug 1;84(3-4):297-316. doi: 10.1016/s0304-4017(99)00037-0. Vet Parasitol. 1999. PMID: 10456420 Review.
Cited by
-
Expression of Heat Shock Protein 70 (HSP70) and Astacin Genes of Strongyloides stercoralis as well as HSP70 and HSP17.1 Genes of S. ratti in Adult and Larval Stages of S. stercoralis.Iran J Parasitol. 2024 Jan-Mar;19(1):1-8. doi: 10.18502/ijpa.v19i1.15187. Iran J Parasitol. 2024. PMID: 38654956 Free PMC article.
-
Functional genomic exploration reveals that Ss-RIOK-1 is essential for the development and survival of Strongyloides stercoralis larvae.Int J Parasitol. 2017 Dec;47(14):933-940. doi: 10.1016/j.ijpara.2017.06.005. Epub 2017 Aug 3. Int J Parasitol. 2017. PMID: 28780152 Free PMC article.
-
Proteomic Analysis of the Excretory and Secretory Proteins of Haemonchus contortus (HcESP) Binding to Goat PBMCs In Vivo Revealed Stage-Specific Binding Profiles.PLoS One. 2016 Jul 28;11(7):e0159796. doi: 10.1371/journal.pone.0159796. eCollection 2016. PLoS One. 2016. PMID: 27467391 Free PMC article.
-
Exploring features and function of Ss-riok-3, an enigmatic kinase gene from Strongyloides stercoralis.Parasit Vectors. 2014 Dec 5;7:561. doi: 10.1186/s13071-014-0561-z. Parasit Vectors. 2014. PMID: 25477034 Free PMC article.
-
The Rio1 protein kinases/ATPases: conserved regulators of growth, division, and genomic stability.Curr Genet. 2019 Apr;65(2):457-466. doi: 10.1007/s00294-018-0912-y. Epub 2018 Dec 4. Curr Genet. 2019. PMID: 30515528 Review.
References
-
- Igra-Siegman Y, Kapila R, Sen P, Kaminski ZC, Louria DB (1981) Syndrome of hyperinfection with Strongyloides stercoralis . Rev Infect Dis 3: 397–407. - PubMed
-
- Schad G (1989) Morphology and life history of Strongyloides stercoralis. Strongyloidiasis: a major roundworm infection of man. Philadelphia: Taylor & Francis 85–104.
-
- Hanks SK, Quinn AM, Hunter T (1988) The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241: 42–52. - PubMed
-
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S (2002) The protein kinase complement of the human genome. Science 298: 1912–1934. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

