Potentiator ivacaftor abrogates pharmacological correction of ΔF508 CFTR in cystic fibrosis
- PMID: 25101886
- PMCID: PMC4272825
- DOI: 10.1126/scitranslmed.3008680
Potentiator ivacaftor abrogates pharmacological correction of ΔF508 CFTR in cystic fibrosis
Abstract
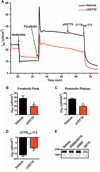
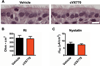
Cystic fibrosis (CF) is caused by mutations in the CF transmembrane conductance regulator (CFTR). Newly developed "correctors" such as lumacaftor (VX-809) that improve CFTR maturation and trafficking and "potentiators" such as ivacaftor (VX-770) that enhance channel activity may provide important advances in CF therapy. Although VX-770 has demonstrated substantial clinical efficacy in the small subset of patients with a mutation (G551D) that affects only channel activity, a single compound is not sufficient to treat patients with the more common CFTR mutation, ΔF508. Thus, patients with ΔF508 will likely require treatment with both correctors and potentiators to achieve clinical benefit. However, whereas the effectiveness of acute treatment with this drug combination has been demonstrated in vitro, the impact of chronic therapy has not been established. In studies of human primary airway epithelial cells, we found that both acute and chronic treatment with VX-770 improved CFTR function in cells with the G551D mutation, consistent with clinical studies. In contrast, chronic VX-770 administration caused a dose-dependent reversal of VX-809-mediated CFTR correction in ΔF508 homozygous cultures. This result reflected the destabilization of corrected ΔF508 CFTR by VX-770, markedly increasing its turnover rate. Chronic VX-770 treatment also reduced mature wild-type CFTR levels and function. These findings demonstrate that chronic treatment with CFTR potentiators and correctors may have unexpected effects that cannot be predicted from short-term studies. Combining these drugs to maximize rescue of ΔF508 CFTR may require changes in dosing and/or development of new potentiator compounds that do not interfere with CFTR stability.
Copyright © 2014, American Association for the Advancement of Science.
Figures





Similar articles
-
Some gating potentiators, including VX-770, diminish ΔF508-CFTR functional expression.Sci Transl Med. 2014 Jul 23;6(246):246ra97. doi: 10.1126/scitranslmed.3008889. Sci Transl Med. 2014. PMID: 25101887 Free PMC article.
-
Cystic fibrosis transmembrane conductance regulator (CFTR) potentiators protect G551D but not ΔF508 CFTR from thermal instability.Biochemistry. 2014 Sep 9;53(35):5613-8. doi: 10.1021/bi501007v. Epub 2014 Aug 22. Biochemistry. 2014. PMID: 25148434 Free PMC article.
-
Ivacaftor: the first therapy acting on the primary cause of cystic fibrosis.Drugs Today (Barc). 2013 Apr;49(4):253-60. doi: 10.1358/dot.2013.49.4.1940984. Drugs Today (Barc). 2013. PMID: 23616952
-
Cystic fibrosis transmembrane conductance regulator-modifying medications: the future of cystic fibrosis treatment.Ann Pharmacother. 2012 Jul-Aug;46(7-8):1065-75. doi: 10.1345/aph.1R076. Epub 2012 Jun 26. Ann Pharmacother. 2012. PMID: 22739718 Review.
-
The preclinical discovery and development of the combination of ivacaftor + tezacaftor used to treat cystic fibrosis.Expert Opin Drug Discov. 2020 Aug;15(8):873-891. doi: 10.1080/17460441.2020.1750592. Epub 2020 Apr 15. Expert Opin Drug Discov. 2020. PMID: 32290721 Review.
Cited by
-
Modulator Combination Improves In Vitro the Microrheological Properties of the Airway Surface Liquid of Cystic Fibrosis Airway Epithelia.Int J Mol Sci. 2022 Sep 27;23(19):11396. doi: 10.3390/ijms231911396. Int J Mol Sci. 2022. PMID: 36232697 Free PMC article.
-
Identification, Structure-Activity Relationship, and Biological Characterization of 2,3,4,5-Tetrahydro-1H-pyrido[4,3-b]indoles as a Novel Class of CFTR Potentiators.J Med Chem. 2020 Oct 8;63(19):11169-11194. doi: 10.1021/acs.jmedchem.0c01050. Epub 2020 Sep 18. J Med Chem. 2020. PMID: 32946228 Free PMC article.
-
From the endoplasmic reticulum to the plasma membrane: mechanisms of CFTR folding and trafficking.Cell Mol Life Sci. 2017 Jan;74(1):39-55. doi: 10.1007/s00018-016-2387-7. Epub 2016 Oct 3. Cell Mol Life Sci. 2017. PMID: 27699454 Free PMC article. Review.
-
Cystic Fibrosis: The Dawn of a New Therapeutic Era.Am J Respir Crit Care Med. 2017 Apr 15;195(8):979-984. doi: 10.1164/rccm.201606-1250PP. Am J Respir Crit Care Med. 2017. PMID: 27710011 Free PMC article. No abstract available.
-
Molecular Mechanism of Action of Trimethylangelicin Derivatives as CFTR Modulators.Front Pharmacol. 2018 Jul 4;9:719. doi: 10.3389/fphar.2018.00719. eCollection 2018. Front Pharmacol. 2018. PMID: 30022950 Free PMC article.
References
-
- Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2004;23:146–158. - PubMed
-
- Accurso FJ. Early pulmonary disease in cystic fibrosis. Current opinion in pulmonary medicine. 1997;3:400–403. - PubMed
-
- Davis PB. Cystic fibrosis since 1938. American journal of respiratory and critical care medicine. 2006;173:475–482. - PubMed
-
- Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui LC. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–1080. - PubMed
-
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

