Modulation of PPARγ provides new insights in a stress induced premature senescence model
- PMID: 25101957
- PMCID: PMC4125176
- DOI: 10.1371/journal.pone.0104045
Modulation of PPARγ provides new insights in a stress induced premature senescence model
Abstract
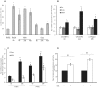
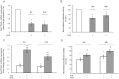
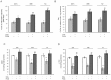
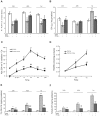
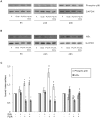
Peroxisome proliferator-activated receptor gamma (PPARγ) may be involved in a key mechanism of the skin aging process, influencing several aspects related to the age-related degeneration of skin cells, including antioxidant unbalance. Therefore, we investigated whether the up-modulation of this nuclear receptor exerts a protective effect in a stress-induced premature senescence (SIPS) model based on a single exposure of human dermal fibroblasts to 8-methoxypsoralen plus + ultraviolet-A-irradiation (PUVA). Among possible PPARγ modulators, we selected 2,4,6-octatrienoic acid (Octa), a member of the parrodiene family, previously reported to promote melanogenesis and antioxidant defense in normal human melanocytes through a mechanism involving PPARγ activation. Exposure to PUVA induced an early and significant decrease in PPARγ expression and activity. PPARγ up-modulation counteracted the antioxidant imbalance induced by PUVA and reduced the expression of stress response genes with a synergistic increase of different components of the cell antioxidant network, such as catalase and reduced glutathione. PUVA-treated fibroblasts grown in the presence of Octa are partially but significantly rescued from the features of the cellular senescence-like phenotype, such as cytoplasmic enlargement, the expression of senescence-associated-β-galactosidase, matrix-metalloproteinase-1, and cell cycle proteins. Moreover, the alterations in the cell membrane lipids, such as the decrease in the polyunsaturated fatty acid content of phospholipids and the increase in cholesterol levels, which are typical features of cell aging, were prevented. Our data suggest that PPARγ is one of the targets of PUVA-SIPS and that its pharmacological up-modulation may represent a novel therapeutic approach for the photooxidative skin damage.
Conflict of interest statement
Figures




Similar articles
-
The activation of PPARγ by 2,4,6-Octatrienoic acid protects human keratinocytes from UVR-induced damages.Sci Rep. 2017 Aug 23;7(1):9241. doi: 10.1038/s41598-017-09578-3. Sci Rep. 2017. PMID: 28835664 Free PMC article.
-
Azelaic acid reduced senescence-like phenotype in photo-irradiated human dermal fibroblasts: possible implication of PPARγ.Exp Dermatol. 2013 Jan;22(1):41-7. doi: 10.1111/exd.12066. Exp Dermatol. 2013. PMID: 23278893
-
2,4,6-Octatrienoic acid is a novel promoter of melanogenesis and antioxidant defence in normal human melanocytes via PPAR-γ activation.Pigment Cell Melanoma Res. 2011 Aug;24(4):618-30. doi: 10.1111/j.1755-148X.2011.00887.x. Epub 2011 Aug 11. Pigment Cell Melanoma Res. 2011. PMID: 21762468
-
Psoralen plus UVA (PUVA) induced premature senescence as a model for stress-induced premature senescence.Exp Gerontol. 2002 Oct-Nov;37(10-11):1197-201. doi: 10.1016/s0531-5565(02)00143-2. Exp Gerontol. 2002. PMID: 12470831 Review.
-
Photoaging as a consequence of natural and therapeutic ultraviolet irradiation--studies on PUVA-induced senescence-like growth arrest of human dermal fibroblasts.Exp Gerontol. 2003 Nov-Dec;38(11-12):1265-70. doi: 10.1016/j.exger.2003.09.006. Exp Gerontol. 2003. PMID: 14698806 Review.
Cited by
-
The activation of PPARγ by 2,4,6-Octatrienoic acid protects human keratinocytes from UVR-induced damages.Sci Rep. 2017 Aug 23;7(1):9241. doi: 10.1038/s41598-017-09578-3. Sci Rep. 2017. PMID: 28835664 Free PMC article.
-
Adipose tissue-derived extracellular fraction characterization: biological and clinical considerations in regenerative medicine.Stem Cell Res Ther. 2018 Aug 9;9(1):207. doi: 10.1186/s13287-018-0956-4. Stem Cell Res Ther. 2018. PMID: 30092820 Free PMC article.
-
Antioxidant activity of mycelia methanolic extracts of endophytic fungi BvFV and BvFIX isolated from leaves of Bauhinia variegata.Front Fungal Biol. 2022 Dec 2;3:1048734. doi: 10.3389/ffunb.2022.1048734. eCollection 2022. Front Fungal Biol. 2022. PMID: 37746214 Free PMC article.
-
Sex hormone binding globulin (SHBG) modulates mitochondrial dynamics in PPARγ-depleted equine adipose derived stromal cells.J Mol Med (Berl). 2024 Aug;102(8):1015-1036. doi: 10.1007/s00109-024-02459-z. Epub 2024 Jun 14. J Mol Med (Berl). 2024. PMID: 38874666 Free PMC article.
-
The PPARγ-SETD8 axis constitutes an epigenetic, p53-independent checkpoint on p21-mediated cellular senescence.Aging Cell. 2017 Aug;16(4):797-813. doi: 10.1111/acel.12607. Epub 2017 May 17. Aging Cell. 2017. PMID: 28514051 Free PMC article.
References
-
- Halliday GM (2005) Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat Res 571: 107–120. - PubMed
-
- Bruls WA, Van Weedlden H, Van der Leun JC (1984) Transmission of UV-irradiation through human epidermal layers as a factor influencing the minimal erythema dose. Photochem Photobiol 39: 63–67. - PubMed
-
- El-Domyati M, Attia S, Saleh F, Brown D, Birk DE, et al. (2002) Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol 11: 398–405. - PubMed
-
- Yasui H, Sakurai H (2002) Age-dependent generation of reactive oxygen species in the skin of live hairless rats exposed to UVA light. Exp Dermatol 12: 655–661. - PubMed
-
- Cunningham ML, Krinsky NI, Giovanazzi SM, Peak MJ (1985) Superoxide anion is generated from cellular metabolites by solar radiation and its components. Free Radic Biol Med 1: 381–385. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

