Proteomic analysis of the action of the Mycobacterium ulcerans toxin mycolactone: targeting host cells cytoskeleton and collagen
- PMID: 25101965
- PMCID: PMC4125307
- DOI: 10.1371/journal.pntd.0003066
Proteomic analysis of the action of the Mycobacterium ulcerans toxin mycolactone: targeting host cells cytoskeleton and collagen
Abstract
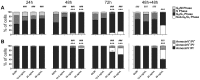
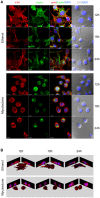
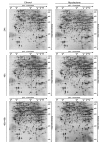
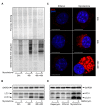
Buruli ulcer (BU) is a neglected tropical disease caused by Mycobacterium ulcerans. The tissue damage characteristic of BU lesions is known to be driven by the secretion of the potent lipidic exotoxin mycolactone. However, the molecular action of mycolactone on host cell biology mediating cytopathogenesis is not fully understood. Here we applied two-dimensional electrophoresis (2-DE) to identify the mechanisms of mycolactone's cellular action in the L929 mouse fibroblast proteome. This revealed 20 changed spots corresponding to 18 proteins which were clustered mainly into cytoskeleton-related proteins (Dync1i2, Cfl1, Crmp2, Actg1, Stmn1) and collagen biosynthesis enzymes (Plod1, Plod3, P4ha1). In line with cytoskeleton conformational disarrangements that are observed by immunofluorescence, we found several regulators and constituents of both actin- and tubulin-cytoskeleton affected upon exposure to the toxin, providing a novel molecular basis for the effect of mycolactone. Consistent with these cytoskeleton-related alterations, accumulation of autophagosomes as well as an increased protein ubiquitination were observed in mycolactone-treated cells. In vivo analyses in a BU mouse model revealed mycolactone-dependent structural changes in collagen upon infection with M. ulcerans, associated with the reduction of dermal collagen content, which is in line with our proteomic finding of mycolactone-induced down-regulation of several collagen biosynthesis enzymes. Our results unveil the mechanisms of mycolactone-induced molecular cytopathogenesis on exposed host cells, with the toxin compromising cell structure and homeostasis by inducing cytoskeleton alterations, as well as disrupting tissue structure, by impairing the extracellular matrix biosynthesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
The Mycobacterium ulcerans toxin mycolactone causes destructive Sec61-dependent loss of the endothelial glycocalyx and vessel basement membrane to drive skin necrosis.Elife. 2025 Feb 6;12:RP86931. doi: 10.7554/eLife.86931. Elife. 2025. PMID: 39913180 Free PMC article.
-
Mycobacterium ulcerans toxic macrolide, mycolactone modulates the host immune response and cellular location of M. ulcerans in vitro and in vivo.Cell Microbiol. 2005 Sep;7(9):1295-304. doi: 10.1111/j.1462-5822.2005.00557.x. Cell Microbiol. 2005. PMID: 16098217
-
The One That Got Away: How Macrophage-Derived IL-1β Escapes the Mycolactone-Dependent Sec61 Blockade in Buruli Ulcer.Front Immunol. 2022 Jan 26;12:788146. doi: 10.3389/fimmu.2021.788146. eCollection 2021. Front Immunol. 2022. PMID: 35154073 Free PMC article.
-
Recent advances: role of mycolactone in the pathogenesis and monitoring of Mycobacterium ulcerans infection/Buruli ulcer disease.Cell Microbiol. 2016 Jan;18(1):17-29. doi: 10.1111/cmi.12547. Cell Microbiol. 2016. PMID: 26572803 Free PMC article. Review.
-
Immunity against Mycobacterium ulcerans: The subversive role of mycolactone.Immunol Rev. 2021 May;301(1):209-221. doi: 10.1111/imr.12956. Epub 2021 Feb 19. Immunol Rev. 2021. PMID: 33607704 Review.
Cited by
-
Mycolactone-mediated neurite degeneration and functional effects in cultured human and rat DRG neurons: Mechanisms underlying hypoalgesia in Buruli ulcer.Mol Pain. 2016 Jun 20;12:1744806916654144. doi: 10.1177/1744806916654144. Print 2016. Mol Pain. 2016. PMID: 27325560 Free PMC article.
-
The potent effect of mycolactone on lipid membranes.PLoS Pathog. 2018 Jan 10;14(1):e1006814. doi: 10.1371/journal.ppat.1006814. eCollection 2018 Jan. PLoS Pathog. 2018. PMID: 29320578 Free PMC article.
-
Clinical Epidemiology of Buruli Ulcer from Benin (2005-2013): Effect of Time-Delay to Diagnosis on Clinical Forms and Severe Phenotypes.PLoS Negl Trop Dis. 2015 Sep 10;9(9):e0004005. doi: 10.1371/journal.pntd.0004005. eCollection 2015. PLoS Negl Trop Dis. 2015. PMID: 26355838 Free PMC article.
-
Heterogeneous Family of Cyclomodulins: Smart Weapons That Allow Bacteria to Hijack the Eukaryotic Cell Cycle and Promote Infections.Front Cell Infect Microbiol. 2017 May 23;7:208. doi: 10.3389/fcimb.2017.00208. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28589102 Free PMC article. Review.
-
Mycolactone causes destructive Sec61-dependent loss of the endothelial glycocalyx and vessel basement membrane: a new indirect mechanism driving tissue necrosis in Mycobacterium ulcerans infection.bioRxiv [Preprint]. 2024 Oct 1:2023.02.21.529382. doi: 10.1101/2023.02.21.529382. bioRxiv. 2024. Update in: Elife. 2025 Feb 06;12:RP86931. doi: 10.7554/eLife.86931. PMID: 36865118 Free PMC article. Updated. Preprint.
References
-
- MacCallum P, Tolhurst JC, Sìssons HA (1948) A new mycobacterial infection in man. J Pathol Bacteriol 60: 93–122. - PubMed
-
- Connor DH, Lunn HF (1965) Mycobacterium ulcerans infection (with comments on pathogenesis). Int J Lepr 33 Suppl: 698–709. - PubMed
-
- Silva MT, Portaels F, Pedrosa J (2009) Pathogenetic mechanisms of the intracellular parasite Mycobacterium ulcerans leading to Buruli ulcer. Lancet Infect Dis 9: 699–710. - PubMed
-
- Connor DH, Lunn HF (1966) Buruli Ulceration: A clinicopathologic study of 38 Ugandans with Mycobacterium ulcerans ulceration. Arch Pathol 81: 183–199.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

