In vitro anti-influenza virus activities of a new lignan glycoside from the latex of Calotropis gigantea
- PMID: 25102000
- PMCID: PMC4125211
- DOI: 10.1371/journal.pone.0104544
In vitro anti-influenza virus activities of a new lignan glycoside from the latex of Calotropis gigantea
Abstract
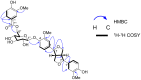
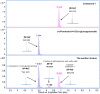
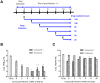
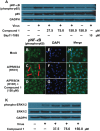
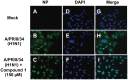
A new lignan glycoside, (+)-pinoresinol 4-O-[6″-O-vanilloyl]-β-D-glucopyranoside (1) and two known phenolic compounds, 6'-O-vanilloyltachioside (2) and 6'-O-vanilloylisotachioside (3) were isolated from the latex of Calotropis gigantea (Asclepiadaceae). The structure of the new compound was elucidated by using spectroscopic and chemical methods. Three isolates (1-3) and one authentic compound, (+)-pinoresinol 4-O-β-D-glucopyranoside, were screened for A/PR/8/34 (H1N1) inhibitory activity by cytopathic effect (CPE) inhibition assay on MDCK cells. Compound 1 showed inhibitory activity against A/PR/8/34 (H1N1). In sharp contrast, the other three compounds (2, 3 and (+)-pinoresinol 4-O-β-D-glucopyranoside) did not show such activity. An analysis of structure-activity relationship between 1 and (+)-pinoresinol 4-O-β-D-glucopyranoside revealed that the presence of a vanilloyl group in the sugar moiety of 1 is crucial for its anti-influenza virus activity. Compound 1 was further evaluated for in vitro inhibitory activities against a panel of human and avian influenza viruses by CPE inhibition assay. It showed inhibitory effect against human influenza viruses in both subtypes A and B (IC50 values around 13.4-39.8 µM with SI values of 3.7-11.4), while had no effect on avian influenza viruses. Its antiviral activity against human influenza viruses subtype A was further confirmed by plaque reduction assay. The time course assay indicated that 1 exerts its antiviral activity at the early stage of viral replication. A mechanistic study showed that 1 efficiently inhibited influenza virus-induced activation of NF-κB pathway in a dose-dependent manner, but had no effect on virus-induced activation of Raf/MEK/ERK pathway. Further studies demonstrated that nuclear translocation of transcription factor NF-κB induced by influenza virus was significantly blocked by 1, meanwhile, nuclear export of viral ribonucleoproteins was also effectively inhibited. These findings suggest that this new lignan glycoside from Calotropis gigantea, may have therapeutic potential in influenza virus infection through inhibition of NF-κB pathway and viral ribonucleoproteins nuclear export.
Conflict of interest statement
Figures



References
-
- World Health Organization (2014) Available: http://www.who.int/topics/influenza/en. Accessed: 2014 Apr 30.
-
- Gao HN, Lu HZ, Cao B, Du B, Shang H, et al. (2013) Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med 368: 2277–2285. - PubMed
-
- Gao GF (2014) Influenza and the live poultry trade. Science 344: 235. - PubMed
-
- Deyde VM, Xu X, Bright RA, Shaw M, Smith CB, et al. (2007) Surveillance of resistance to adamantanes among influenza A(H3N2) and A(H1N1) viruses isolated worldwide. J Infect Dis 196: 249–257. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

