The Mysterious Ways of ErbB2/HER2 Trafficking
- PMID: 25102001
- PMCID: PMC4194043
- DOI: 10.3390/membranes4030424
The Mysterious Ways of ErbB2/HER2 Trafficking
Abstract
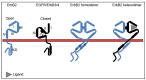
The EGFR- or ErbB-family of receptor tyrosine kinases consists of EGFR/ErbB1, ErbB2/HER2, ErbB3/HER3 and ErbB4/HER4. Receptor activation and downstream signaling are generally initiated upon ligand-induced receptor homo- or heterodimerization at the plasma membrane, and endocytosis and intracellular membrane transport are crucial for regulation of the signaling outcome. Among the receptors, ErbB2 is special in several ways. Unlike the others, ErbB2 has no known ligand, but is still the favored dimerization partner. Furthermore, while the other receptors are down-regulated either constitutively or upon ligand-binding, ErbB2 is resistant to down-regulation, and also inhibits down-regulation of its partner upon heterodimerization. The reason(s) why ErbB2 is resistant to down-regulation are the subject of debate. Contrary to other ErbB-proteins, mature ErbB2 needs Hsp90 as chaperone. Several data suggest that Hsp90 is an important regulator of factors like ErbB2 stability, dimerization and/or signaling. Hsp90 inhibitors induce degradation of ErbB2, but whether Hsp90 directly makes ErbB2 endocytosis resistant is unclear. Exposure to anti-ErbB2 antibodies can also induce down-regulation of ErbB2. Down-regulation induced by Hsp90 inhibitors or antibodies does at least partly involve internalization and endosomal sorting to lysosomes for degradation, but also retrograde trafficking to the nucleus has been reported. In this review, we will discuss different molecular mechanisms suggested to be important for making ErbB2 resistant to down-regulation, and review how membrane trafficking is involved when down-regulation and/or relocalization of ErbB2 is induced.
Figures


References
-
- Garrett T.P., McKern N.M., Lou M., Elleman T.C., Adams T.E., Lovrecz G.O., Kofler M., Jorissen R.N., Nice E.C., Burgess A.W., et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol. Cell. 2003;11:495–505. doi: 10.1016/S1097-2765(03)00048-0. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

