Neuropathogenesis of Japanese encephalitis in a primate model
- PMID: 25102067
- PMCID: PMC4125110
- DOI: 10.1371/journal.pntd.0002980
Neuropathogenesis of Japanese encephalitis in a primate model
Abstract
Background: Japanese encephalitis (JE) is a major cause of mortality and morbidity for which there is no treatment. In addition to direct viral cytopathology, the inflammatory response is postulated to contribute to the pathogenesis. Our goal was to determine the contribution of bystander effects and inflammatory mediators to neuronal cell death.
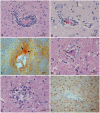
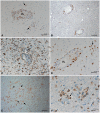
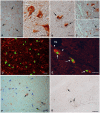
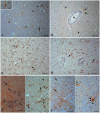
Methodology/principal findings: Material from a macaque model was used to characterize the inflammatory response and cytopathic effects of JE virus (JEV). Intranasal JEV infection induced a non-suppurative encephalitis, dominated by perivascular, infiltrates of mostly T cells, alongside endothelial cell activation, vascular damage and blood brain barrier (BBB) leakage; in the adjacent parenchyma there was macrophage infiltration, astrocyte and microglia activation. JEV antigen was mostly in neurons, but there was no correlation between intensity of viral infection and degree of inflammatory response. Apoptotic cell death occurred in both infected and non-infected neurons. Interferon-α, which is a microglial activator, was also expressed by both. Tumour Necrosis Factor-α, inducible nitric oxide synthase and nitrotyrosine were expressed by microglial cells, astrocytes and macrophages. The same cells expressed matrix metalloproteinase (MMP)-2 whilst MMP-9 was expressed by neurons.
Conclusions/significance: The results are consistent with JEV inducing neuronal apoptotic death and release of cytokines that initiate microglial activation and release of pro-inflammatory and apoptotic mediators with subsequent apoptotic death of both infected and uninfected neurons. Activation of astrocytes, microglial and endothelial cells likely contributes to inflammatory cell recruitment and BBB breakdown. It appears that neuronal apoptotic death and activation of microglial cells and astrocytes play a crucial role in the pathogenesis of JE.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Solomon T, Vaughn DW (2002) Pathogenesis and clinical features of Japanese encephalitis and West Nile virus infections. Curr Top Microbiol Immunol 267: 171–194. - PubMed
-
- Ghoshal A, Das S, Ghosh S, Mishra MK, Sharma V, et al. (2007) Proinflammatory mediators released by activated microglia induces neuronal death in Japanese encephalitis. Glia 55: 483–496. - PubMed
-
- Das S, Mishra MK, Ghosh J, Basu A (2008) Japanese Encephalitis Virus infection induces IL-18 and IL-1beta in microglia and astrocytes: correlation with in vitro cytokine responsiveness of glial cells and subsequent neuronal death. J Neuroimmunol 195: 60–72. - PubMed
-
- Myint KS, Raengsakulrach B, Young D, Gettayacamin M, Ferguson LM, et al. (1999) Production of lethal infection that resembles fatal human disease by intranasal inoculation of macaques with Japanese encephalitis virus. Am J Trop Med Hyg 60: 338–342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

