Polarized exocyst-mediated vesicle fusion directs intracellular lumenogenesis within the C. elegans excretory cell
- PMID: 25102190
- PMCID: PMC4373406
- DOI: 10.1016/j.ydbio.2014.07.019
Polarized exocyst-mediated vesicle fusion directs intracellular lumenogenesis within the C. elegans excretory cell
Abstract
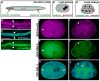
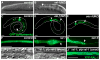
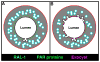
Lumenogenesis of small seamless tubes occurs through intracellular membrane growth and directed vesicle fusion events. Within the Caenorhabditis elegans excretory cell, which forms seamless intracellular tubes (canals) that mediate osmoregulation, lumens grow in length and diameter when vesicles fuse with the expanding lumenal surface. Here, we show that lumenal vesicle fusion depends on the small GTPase RAL-1, which localizes to vesicles and acts through the exocyst vesicle-tethering complex. Loss of either the exocyst or RAL-1 prevents excretory canal lumen extension. Within the excretory canal and other polarized cells, the exocyst co-localizes with the PAR polarity proteins PAR-3, PAR-6 and PKC-3. Using early embryonic cells to determine the functional relationships between the exocyst and PAR proteins, we show that RAL-1 recruits the exocyst to the membrane, while PAR proteins concentrate membrane-localized exocyst proteins to a polarized domain. These findings reveal that RAL-1 and the exocyst direct the polarized vesicle fusion events required for intracellular lumenogenesis of the excretory cell, suggesting mechanistic similarities in the formation of topologically distinct multicellular and intracellular lumens.
Keywords: Exocyst; Lumenogenesis; Osmoregulation; PAR proteins; Tubulogenesis; Vesicle trafficking.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

