Convergent and divergent mechanisms of sugar recognition across kingdoms
- PMID: 25102772
- PMCID: PMC4444583
- DOI: 10.1016/j.sbi.2014.07.003
Convergent and divergent mechanisms of sugar recognition across kingdoms
Abstract
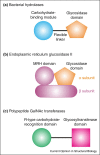
Protein modules that bind specific oligosaccharides are found across all kingdoms of life from single-celled organisms to man. Different, overlapping and evolving designations for sugar-binding domains in proteins can sometimes obscure common features that often reflect convergent solutions to the problem of distinguishing sugars with closely similar structures and binding them with sufficient affinity to achieve biologically meaningful results. Structural and functional analysis has revealed striking parallels between protein domains with widely different structures and evolutionary histories that employ common solutions to the sugar recognition problem. Recent studies also demonstrate that domains descended from common ancestors through divergent evolution appear more widely across the kingdoms of life than had previously been recognized.
Copyright © 2014 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Sharon N., Lis H. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology. 2004;14:53R–62R. - PubMed
-
- Etzold S., Juge N. Structural insights into bacterial recognition of intestinal mucins. Curr Opin Struct Biol. 2014;28 (in press) - PubMed
-
- Abbott W.D., van Bueren A.L. Using structure to inform carbohydrate binding module function. Curr Opin Struct Biol. 2014;28 (in press) - PubMed
-
- Drickamer K., Taylor M.E. Identification of lectins from genomic sequence data. Methods Enzymol. 2003;362:592–599. - PubMed
-
- Weis W.I., Taylor M.E., Drickamer K. The C-type lectin superfamily in the immune system. Immunol Rev. 1998;163:19–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

