Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies
- PMID: 25103255
- PMCID: PMC4455970
- DOI: 10.1038/nrd4363
Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies
Abstract
The formulation and delivery of biopharmaceutical drugs, such as monoclonal antibodies and recombinant proteins, poses substantial challenges owing to their large size and susceptibility to degradation. In this Review we highlight recent advances in formulation and delivery strategies--such as the use of microsphere-based controlled-release technologies, protein modification methods that make use of polyethylene glycol and other polymers, and genetic manipulation of biopharmaceutical drugs--and discuss their advantages and limitations. We also highlight current and emerging delivery routes that provide an alternative to injection, including transdermal, oral and pulmonary delivery routes. In addition, the potential of targeted and intracellular protein delivery is discussed.
Figures



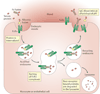


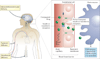
References
-
- Biotech products in big pharma clinical pipelines have grown dramatically. Tufts CSDD Impact Report. 2013;15:1–4. [No authors listed.]
-
- Pharmaceutical Research and Manufacturers of America. Medicines in Development — Biologics (2013 report) PhRMA. 2013 [online], http://www.phrma.org/sites/default/files/pdf/biologics2013.pdf.
-
- Langer R, Folkman J. Polymers for the sustained release of proteins and other macromolecules. Nature. 1976;263:797–800. - PubMed
-
- Cohen S, Yoshioka T, Lucarelli M, Hwang LH, Langer R. Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres. Pharm. Res. 1991;8:713–720. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

