Differential effects of caveolin-1 and -2 knockdown on aqueous outflow and altered extracellular matrix turnover in caveolin-silenced trabecular meshwork cells
- PMID: 25103269
- PMCID: PMC4152152
- DOI: 10.1167/iovs.14-14519
Differential effects of caveolin-1 and -2 knockdown on aqueous outflow and altered extracellular matrix turnover in caveolin-silenced trabecular meshwork cells
Abstract
Purpose: A single nucleotide polymorphism (SNP) identified between caveolin-1 (CAV1) and caveolin-2 (CAV2) on chromosome 7 is associated with glaucoma. One function of CAVs is endocytosis and recycling of extracellular matrix (ECM) components. Here, we generated CAV-silencing lentivirus to evaluate the effects on ECM turnover by trabecular meshwork (TM) cells and to measure the effect on outflow facility in anterior segment perfusion culture.
Methods: Short hairpin CAV1 and CAV2 silencing and control lentivirus were generated, characterized, and applied to anterior segments in perfusion culture. Colocalization of CAVs with various ECM molecules in TM cells was investigated using immunofluorescence and confocal microscopy. Western immunoblotting and fluorogenic-based enzyme activity assays were used to investigate ECM protein levels and degradation, respectively.
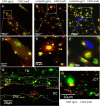
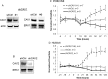
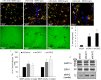
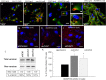
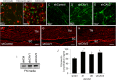
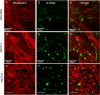
Results: Endogenous CAVs colocalized with cortactin at podosome- or invadopodia-like structures (PILS), which are areas of focal ECM degradation. In perfusion culture, outflow rates increased significantly in CAV1-silenced anterior segments, whereas outflow significantly decreased in CAV2-silenced anterior segments. Matrix metalloproteinase (MMP)2 and MMP14, and a disintegrin and metalloproteinase with thrombospondin motifs-4 (ADAMTS4) colocalized with both CAVs in TM cells. Protein levels and enzyme activities of MMP/ADAMTS4, fibronectin protein levels, actin stress fibers, and α-smooth muscle actin were all increased in CAV-silenced cells.
Conclusions: Caveolin-mediated endocytosis is one mechanism by which TM cells can alter the physiological catabolism of ECM in order to change the composition of the outflow channels in the TM to regulate aqueous outflow resistance. Dysregulation of CAV function could contribute to the pathological changes in ECM that are observed in glaucoma.
Keywords: endocytosis; extracellular matrix; glaucoma anterior segment; matrix metalloproteinases; trabecular meshwork.
Copyright 2014 The Association for Research in Vision and Ophthalmology, Inc.
Figures
References
-
- Boland MV, Quigley HA. Risk factors and open-angle glaucoma: classification and application. J Glaucoma. 2007; 16: 406–418 - PubMed
-
- Rezaie T, Child A, Hitchings R, et al. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 2002; 295: 1077–1079 - PubMed
-
- Stone EM, Fingert JH, Alward WL, et al. Identification of a gene that causes primary open angle glaucoma. Science. 1997; 275: 668–670 - PubMed
-
- Monemi S, Spaeth G, DaSilva A, et al. Identification of a novel adult-onset primary open-angle glaucoma (POAG) gene on 5q22.1. Hum Mol Genet. 2005; 14: 725–733 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

