Population effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae and non-typeable Haemophilus influenzae in Kilifi, Kenya: findings from cross-sectional carriage studies
- PMID: 25103393
- PMCID: PMC5628631
- DOI: 10.1016/S2214-109X(14)70224-4
Population effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae and non-typeable Haemophilus influenzae in Kilifi, Kenya: findings from cross-sectional carriage studies
Abstract
Background: The effect of 7-valent pneumococcal conjugate vaccine (PCV) in developed countries was enhanced by indirect protection of unvaccinated individuals, mediated by reduced nasopharyngeal carriage of vaccine-serotype pneumococci. The potential indirect protection of 10-valent PCV (PCV10) in a developing country setting is unknown. We sought to estimate the effectiveness of introduction of PCV10 in Kenya against carriage of vaccine serotypes and its effect on other bacteria.
Methods: PCV10 was introduced into the infant vaccination programme in Kenya in January, 2011, accompanied by a catch-up campaign in Kilifi County for children aged younger than 5 years. We did annual cross-sectional carriage studies among an age-stratified, random population sample in the 2 years before and 2 years after PCV10 introduction. A nasopharyngeal rayon swab specimen was collected from each participant and was processed in accordance with WHO recommendations. Prevalence ratios of carriage before and after introduction of PCV10 were calculated by log-binomial regression.
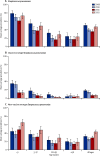
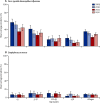
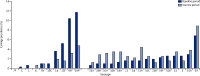
Findings: About 500 individuals were enrolled each year (total n=2031). Among children younger than 5 years, the baseline (2009-10) carriage prevalence was 34% for vaccine-serotype Streptococcus pneumoniae, 41% for non-vaccine-serotype Streptococcus pneumoniae, and 54% for non-typeable Haemophilus influenzae. After PCV10 introduction (2011-12), these percentages were 13%, 57%, and 40%, respectively. Adjusted prevalence ratios were 0·36 (95% CI 0·26-0·51), 1·37 (1·13-1·65), and 0·62 (0·52-0·75), respectively. Among individuals aged 5 years or older, the adjusted prevalence ratios for vaccine-serotype and non-vaccine-serotype S pneumoniae carriage were 0·34 (95% CI 0·18-0·62) and 1·13 (0·92-1·38), respectively. There was no change in prevalence ratio for Staphylococcus aureus (adjusted prevalence ratio for those <5 years old 1·02, 95% CI 0·52-1·99, and for those ≥5 years old 0·90, 0·60-1·35).
Interpretation: After programmatic use of PCV10 in Kilifi, carriage of vaccine serotypes was reduced by two-thirds both in children younger than 5 years and in older individuals. These findings suggest that PCV10 introduction in Africa will have substantial indirect effects on invasive pneumococcal disease.
Funding: GAVI Alliance and Wellcome Trust.
Copyright © 2014 Hammitt et al. Open Access article distributed under the terms of CC BY. Published by .. All rights reserved.
Figures
Comment in
-
Herd protection induced by pneumococcal conjugate vaccine.Lancet Glob Health. 2014 Jul;2(7):e365-6. doi: 10.1016/S2214-109X(14)70241-4. Epub 2014 May 28. Lancet Glob Health. 2014. PMID: 25103373 No abstract available.
References
-
- Lehmann D, Willis J, Moore HC. The changing epidemiology of invasive pneumococcal disease in aboriginal and non-aboriginal western Australians from 1997 through 2007 and emergence of nonvaccine serotypes. Clin Infect Dis. 2010;50:1477–1486. - PubMed
-
- Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;11:760–768. - PubMed
-
- Pilishvili T, Lexau C, Farley MM. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

