Structure of an agonist-bound ionotropic glutamate receptor
- PMID: 25103407
- PMCID: PMC4383034
- DOI: 10.1126/science.1256508
Structure of an agonist-bound ionotropic glutamate receptor
Abstract
Ionotropic glutamate receptors (iGluRs) mediate most excitatory neurotransmission in the central nervous system and function by opening their ion channel in response to binding of agonist glutamate. Here, we report a structure of a homotetrameric rat GluA2 receptor in complex with partial agonist (S)-5-nitrowillardiine. Comparison of this structure with the closed-state structure in complex with competitive antagonist ZK 200775 suggests conformational changes that occur during iGluR gating. Guided by the structures, we engineered disulfide cross-links to probe domain interactions that are important for iGluR gating events. The combination of structural information, kinetic modeling, and biochemical and electrophysiological experiments provides insight into the mechanism of iGluR gating.
Copyright © 2014, American Association for the Advancement of Science.
Figures
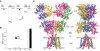


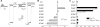
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

