Selective activity over a constitutively active RET-variant of the oral multikinase inhibitor dovitinib: results of the CNIO-BR002 phase I-trial
- PMID: 25103625
- PMCID: PMC5528587
- DOI: 10.1016/j.molonc.2014.07.005
Selective activity over a constitutively active RET-variant of the oral multikinase inhibitor dovitinib: results of the CNIO-BR002 phase I-trial
Abstract
Background: Given our preclinical data showing synergy between dovitinib and paclitaxel in preclinical models we conducted this phase I trial aiming to define the recommended phase II-dose (RP2D) on the basis of toxicity and pharmacodynamic criteria while searching for genetic variants that could sensitize patients to the regimen under study.
Patients and methods: A 3+3 escalation schedule was adopted. Seriated FGF23 and dovitinib and paclitaxel pharmacokinetic profiles were determined along a single-agent dovitinib "priming-phase" followed by a dovitinib + paclitaxel combination phase. RECIST 1.1 criteria and NCI CTCAE V.4.0 were used. In fresh pre-treatment tumor biopsy samples, FGFR1, 2 and 3 amplifications were revealed by FISH probes; 32 missense variants were genotyped in tumors and peripheral blood mononuclear cells with Taqman genotyping assays (FGFR1-3 and RET). Constructs encoding for wild-type and variant genes associated with clinical benefit were transfected into HEK-293 cells for preclinical experiments checking constitutive activation and dovitinib sensitivity of the variants.
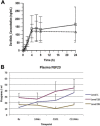
Results: twelve patients were recruited in three dose-levels. At level 1B (200 mg dovitinib 5-days-on/2-days-off plus 60 mg/m 2-week of paclitaxel) more than 50% FGF23 upregulation was observed and no dose-limiting-toxicities (DLTs) occurred. The most frequent toxicities were asthenia, neutropenia, nausea/vomiting and transaminitis. Two patients with progressive disease prior to trial inclusion achieved prolonged disease stabilization. Both had the germline variant G2071A in the RET gene, which led to constitutive activation of the protein product and Y-905 phosphorylation, both in transfectants and in patients with the alteration. This variant was sensitive to dovitinib; in addition both patients experienced progression upon medication withdrawal.
Conclusions: Level 1B was the RP2D as it provided adequate pharmacodynamic exposure to dovitinib. The G2071A germline variant act as a genetic modifier that renders different tumors sensitive to dovitinib.
Keywords: Antiangiogenic; Clinical trial; Dovitinib; Phase I; RET genetic variant; Sensitizing genetic variant.
Copyright © 2014 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Phase I study of dovitinib (TKI258), an oral FGFR, VEGFR, and PDGFR inhibitor, in advanced or metastatic renal cell carcinoma.Clin Cancer Res. 2013 Mar 1;19(5):1257-68. doi: 10.1158/1078-0432.CCR-12-2885. Epub 2013 Jan 21. Clin Cancer Res. 2013. PMID: 23339124 Clinical Trial.
-
Targeting FGFR with dovitinib (TKI258): preclinical and clinical data in breast cancer.Clin Cancer Res. 2013 Jul 1;19(13):3693-702. doi: 10.1158/1078-0432.CCR-13-0190. Epub 2013 May 8. Clin Cancer Res. 2013. PMID: 23658459 Clinical Trial.
-
Phase I/II and pharmacodynamic study of dovitinib (TKI258), an inhibitor of fibroblast growth factor receptors and VEGF receptors, in patients with advanced melanoma.Clin Cancer Res. 2011 Dec 1;17(23):7451-61. doi: 10.1158/1078-0432.CCR-11-1747. Epub 2011 Oct 5. Clin Cancer Res. 2011. PMID: 21976540 Clinical Trial.
-
Phase II results of Dovitinib (TKI258) in patients with metastatic renal cell cancer.Clin Cancer Res. 2014 Jun 1;20(11):3012-22. doi: 10.1158/1078-0432.CCR-13-3006. Epub 2014 Apr 1. Clin Cancer Res. 2014. PMID: 24691021 Clinical Trial.
-
Dovitinib: rationale, preclinical and early clinical data in urothelial carcinoma of the bladder.Expert Opin Investig Drugs. 2014 Nov;23(11):1553-62. doi: 10.1517/13543784.2014.966900. Epub 2014 Oct 4. Expert Opin Investig Drugs. 2014. PMID: 25284004 Review.
Cited by
-
Exploring the Horizon: Anti-Fibroblast Growth Factor Receptor Therapy in Pancreatic Cancer with Aberrant Fibroblast Growth Factor Receptor Expression-A Scoping Review.Cancers (Basel). 2024 Aug 22;16(16):2912. doi: 10.3390/cancers16162912. Cancers (Basel). 2024. PMID: 39199681 Free PMC article.
-
Dovitinib enhances temozolomide efficacy in glioblastoma cells.Mol Oncol. 2017 Aug;11(8):1078-1098. doi: 10.1002/1878-0261.12076. Epub 2017 Jun 5. Mol Oncol. 2017. PMID: 28500786 Free PMC article.
References
-
- Andre, F. , Bachelot, T. , Campone, M. , Dalenc, F. , Perez-Garcia, J.M. , Hurvitz, S.A. , Turner, N. , Rugo, H. , Smith, J.W. , Deudon, S. , Shi, M. , Zhang, Y. , Kay, A. , Porta, D.G. , Yovine, A. , Baselga, J. , 2013. Targeting FGFR with dovitinib (TKI258): preclinical and clinical data in breast cancer. Clin. Cancer Res. 19, 3693–3702. - PubMed
-
- Angevin, E. , Lopez-Martin, J.A. , Lin, C.C. , Gschwend, J.E. , Harzstark, A. , Castellano, D. , Soria, J.C. , Sen, P. , Chang, J. , Shi, M. , Kay, A. , Escudier, B. , 2013. Phase I study of dovitinib (TKI258), an oral FGFR, VEGFR, and PDGFR inhibitor, in advanced or metastatic renal cell carcinoma. Clin. Cancer Res. 19, 1257–1268. - PubMed
-
- Bergh, J. , Bondarenko, I.M. , Lichinitser, M.R. , Liljegren, A. , Greil, R. , Voytko, N.L. , Makhson, A.N. , Cortes, J. , Lortholary, A. , Bischoff, J. , Chan, A. , Delaloge, S. , Huang, X. , Kern, K.A. , Giorgetti, C. , 2012. First-line treatment of advanced breast cancer with sunitinib in combination with docetaxel versus docetaxel alone: results of a prospective, randomized phase III study. J. Clin. Oncol. 30, 921–929. - PubMed
-
- Eisenhauer, E.A. , Therasse, P. , Bogaerts, J. , Schwartz, L.H. , Sargent, D. , Ford, R. , Dancey, J. , Arbuck, S. , Gwyther, S. , Mooney, M. , Rubinstein, L. , Shankar, L. , Dodd, L. , Kaplan, R. , Lacombe, D. , Verweij, J. , 2009. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247. - PubMed
-
- Elisei, R. , Cosci, B. , Romei, C. , Bottici, V. , Sculli, M. , Lari, R. , Barale, R. , Pacini, F. , Pinchera, A. , 2004. RET exon 11 (G691S) polymorphism is significantly more frequent in sporadic medullary thyroid carcinoma than in the general population. J. Clin. Endocrinol. Metab. 89, 3579–3584. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

