T cells targeting a neuronal paraneoplastic antigen mediate tumor rejection and trigger CNS autoimmunity with humoral activation
- PMID: 25103845
- PMCID: PMC4296561
- DOI: 10.1002/eji.201444624
T cells targeting a neuronal paraneoplastic antigen mediate tumor rejection and trigger CNS autoimmunity with humoral activation
Abstract
Paraneoplastic neurologic diseases (PND) involving immune responses directed toward intracellular antigens are poorly understood. Here, we examine immunity to the PND antigen Nova2, which is expressed exclusively in central nervous system (CNS) neurons. We hypothesized that ectopic expression of neuronal antigen in the periphery could incite PND. In our C57BL/6 mouse model, CNS antigen expression limits antigen-specific CD4+ and CD8+ T-cell expansion. Chimera experiments demonstrate that this tolerance is mediated by antigen expression in nonhematopoietic cells. CNS antigen expression does not limit tumor rejection by adoptively transferred transgenic T cells but does limit the generation of a memory population that can be expanded upon secondary challenge in vivo. Despite mediating cancer rejection, adoptively transferred transgenic T cells do not lead to paraneoplastic neuronal targeting. Preliminary experiments suggest an additional requirement for humoral activation to induce CNS autoimmunity. This work provides evidence that the requirements for cancer immunity and neuronal autoimmunity are uncoupled. Since humoral immunity was not required for tumor rejection, B-cell targeting therapy, such as rituximab, may be a rational treatment option for PND that does not hamper tumor immunity.
Keywords: Autoimmunity; Cancer; Cellular immunity; Humoral immunity; Paraneoplastic neurologic disease.
© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors have no conflicting commercial or financial interests.
Figures
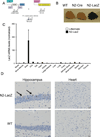

 or Littermate
control
or Littermate
control  mice immunized with b-gal/CFA and PTx. Relative density
was calculated by normalizing to a known commercial b-gal monoclonal antibody.
(B–F) N2-LacZ or Littermate control mice were immunized with AdV-b-gal and PTx.
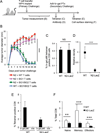
(B) IFNγ ELISPOT responses of splenic CD4+ T cells cultured with irradiated and
peptide pulsed splenocytes 13 days after immunization. (C) Representative FACS plots of
CD8+ and tetramer positive cells 22 days after immunization. Gating strategy presented in
Supporting information Fig. 3A.
(D) Summary of (C), 8 mice per group. (E) IFNγ ELISPOT responses of splenic CD8+ T
cells cultured with EL4 cells pulsed with 1 µM ova (irrelevant), b-gal p96, b-gal
p497, or E22 cells, 13 days after immunization. (F) IFNγ ELISPOT responses of
splenic CD8+ T cells to EL4 cells pulsed with decreasing amounts of b-gal p96 or p497
peptides 13 days post immunization. No IFNγ spots were detected from N2-LacZ mice
at any p96 peptide concentration indicated by
mice immunized with b-gal/CFA and PTx. Relative density
was calculated by normalizing to a known commercial b-gal monoclonal antibody.
(B–F) N2-LacZ or Littermate control mice were immunized with AdV-b-gal and PTx.
(B) IFNγ ELISPOT responses of splenic CD4+ T cells cultured with irradiated and
peptide pulsed splenocytes 13 days after immunization. (C) Representative FACS plots of
CD8+ and tetramer positive cells 22 days after immunization. Gating strategy presented in
Supporting information Fig. 3A.
(D) Summary of (C), 8 mice per group. (E) IFNγ ELISPOT responses of splenic CD8+ T
cells cultured with EL4 cells pulsed with 1 µM ova (irrelevant), b-gal p96, b-gal
p497, or E22 cells, 13 days after immunization. (F) IFNγ ELISPOT responses of
splenic CD8+ T cells to EL4 cells pulsed with decreasing amounts of b-gal p96 or p497
peptides 13 days post immunization. No IFNγ spots were detected from N2-LacZ mice
at any p96 peptide concentration indicated by  . Data are
presented as mean+/−SD of three mice per group unless otherwise indicated. All
data shown is representative of at least 3 experiments. NS indicates difference is not
statistically significant by 2 tailed t-test. * indicates p<0.05, ** indicates
p<0.01, *** indicates p<0.001. Mice exhibited no signs of ataxia, hunched
posturing or death.
. Data are
presented as mean+/−SD of three mice per group unless otherwise indicated. All
data shown is representative of at least 3 experiments. NS indicates difference is not
statistically significant by 2 tailed t-test. * indicates p<0.05, ** indicates
p<0.01, *** indicates p<0.001. Mice exhibited no signs of ataxia, hunched
posturing or death.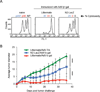


References
-
- Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N. Engl. J. Med. 2003;349:1543–1554. - PubMed
-
- Graus F, Dalmou J, Rene R, Tora M, Malats N, Verschuuren JJ, Cardenal F, et al. Anti-Hu antibodies in patients with small-cell lung cancer: association with complete response to therapy and improved survival. J. Clin. Oncol. 1997;15:2866–2872. - PubMed
-
- Darnell RB, Posner JB. Observing the invisible: successful tumor immunity in humans. Nat. Immunol. 2003;4:201. - PubMed
-
- Darnell RB, DeAngelis LM. Regression of small-cell lung carcinoma in patients with paraneoplastic neuronal antibodies. Lancet. 1993;341:21–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

