Novel inhibitors of human immunodeficiency virus type 2 infectivity
- PMID: 25103850
- PMCID: PMC4233633
- DOI: 10.1099/vir.0.069864-0
Novel inhibitors of human immunodeficiency virus type 2 infectivity
Abstract
Human immunodeficiency virus type 2 (HIV-2) infects about two million people worldwide. HIV-2 has fewer treatment options than HIV-1, yet may evolve drug resistance more quickly. We have analysed several novel drugs for anti-HIV-2 activity. It was observed that 5-azacytidine, clofarabine, gemcitabine and resveratrol have potent anti-HIV-2 activity. The EC50 values for 5-azacytidine, clofarabine and resveratrol were found to be significantly lower with HIV-2 than with HIV-1. A time-of-addition assay was used to analyse the ability of these drugs to interfere with HIV-2 replication. Reverse transcription was the likely target for antiretroviral activity. Taken together, several novel drugs have been discovered to have activity against HIV-2. Based upon their known activities, these drugs may elicit enhanced HIV-2 mutagenesis and therefore be useful for inducing HIV-2 lethal mutagenesis. In addition, the data are consistent with HIV-2 reverse transcriptase being more sensitive than HIV-1 reverse transcriptase to dNTP pool alterations.
© 2014 The Authors.
Figures
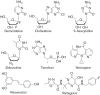
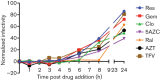
References
-
- Ahn J., Hao C., Yan J., DeLucia M., Mehrens J., Wang C., Gronenborn A. M., Skowronski J. (2012). HIV/simian immunodeficiency virus (SIV) accessory virulence factor Vpx loads the host cell restriction factor SAMHD1 onto the E3 ubiquitin ligase complex CRL4DCAF1. J Biol Chem 287, 12550–12558. 10.1074/jbc.M112.340711 - DOI - PMC - PubMed
-
- Amie S. M., Daly M. B., Noble E., Schinazi R. F., Bambara R. A., Kim B. (2013). Anti-HIV host factor SAMHD1 regulates viral sensitivity to nucleoside reverse transcriptase inhibitors via modulation of cellular deoxyribonucleoside triphosphate (dNTP) levels. J Biol Chem 288, 20683–20691. 10.1074/jbc.M113.472159 - DOI - PMC - PubMed
-
- Andreatta K., Miller M. D., White K. L. (2013). HIV-2 antiviral potency and selection of drug resistance mutations by the integrase strand transfer inhibitor elvitegravir and NRTIs emtricitabine and tenofovir in vitro. J Acquir Immune Defic Syndr 62, 367–374. 10.1097/QAI.0b013e31827b55f1 - DOI - PubMed
-
- Balzarini J. (2004). Current status of the non-nucleoside reverse transcriptase inhibitors of human immunodeficiency virus type 1. Curr Top Med Chem 4, 921–944. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

