Mitochondria regulate neutrophil activation by generating ATP for autocrine purinergic signaling
- PMID: 25104353
- PMCID: PMC4175322
- DOI: 10.1074/jbc.M114.572495
Mitochondria regulate neutrophil activation by generating ATP for autocrine purinergic signaling
Abstract
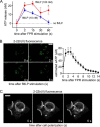
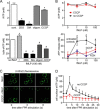
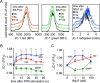
Polymorphonuclear neutrophils (PMNs) form the first line of defense against invading microorganisms. We have shown previously that ATP release and autocrine purinergic signaling via P2Y2 receptors are essential for PMN activation. Here we show that mitochondria provide the ATP that initiates PMN activation. Stimulation of formyl peptide receptors increases the mitochondrial membrane potential (Δψm) and triggers a rapid burst of ATP release from PMNs. This burst of ATP release can be blocked by inhibitors of mitochondrial ATP production and requires an initial formyl peptide receptor-induced Ca(2+) signal that triggers mitochondrial activation. The burst of ATP release generated by the mitochondria fuels a first phase of purinergic signaling that boosts Ca(2+) signaling, amplifies mitochondrial ATP production, and initiates functional PMN responses. Cells then switch to glycolytic ATP production, which fuels a second round of purinergic signaling that sustains Ca(2+) signaling via P2X receptor-mediated Ca(2+) influx and maintains functional PMN responses such as oxidative burst, degranulation, and phagocytosis.
Keywords: ATP; Calcium Signaling; Inflammation; Neutrophil; Purinergic Receptor.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Bodin P., Burnstock G. (2001) Purinergic signalling: ATP release. Neurochem. Res. 26, 959–969 - PubMed
-
- Burnstock G., Verkhratsky A. (2012) in Purinergic Signalling and the Nervous System, pp. 79–118, Springer, Berlin
-
- Ralevic V., Burnstock G. (1998) Receptors for purines and pyrimidines. Pharmacol. Rev. 50, 413–492 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

