Extracellular vesicles as emerging intercellular communicasomes
- PMID: 25104400
- PMCID: PMC4261509
- DOI: 10.5483/bmbrep.2014.47.10.164
Extracellular vesicles as emerging intercellular communicasomes
Abstract
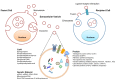
All living cells release extracellular vesicles having pleiotropic functions in intercellular communication. Mammalian extracellular vesicles, also known as exosomes and microvesicles, are spherical bilayered proteolipids composed of various bioactive molecules, including RNAs, DNAs, proteins, and lipids. Extracellular vesicles directly and indirectly control a diverse range of biological processes by transferring membrane proteins, signaling molecules, mRNAs, and miRNAs, and activating receptors of recipient cells. The active interaction of extracellular vesicles with other cells regulates various physiological and pathological conditions, including cancer, infectious diseases, and neurodegenerative disorders. Recent developments in high-throughput proteomics, transcriptomics, and lipidomics tools have provided ample data on the common and specific components of various types of extracellular vesicles. These studies may contribute to the understanding of the molecular mechanism involved in vesicular cargo sorting and the biogenesis of extracellular vesicles, and, further, to the identification of disease-specific biomarkers. This review focuses on the components, functions, and therapeutic and diagnostic potential of extracellular vesicles under various pathophysiological conditions.
Figures

References
-
- Choi D. S., Kim D. K., Kim Y. K., Gho Y. S. Proteomics of extracellular vesicles: Exosomes and ectosomes. Mass Spectrom. Rev. (2014) [Epub ahead of print]. - PubMed
-
- Lee E. Y., Choi D. Y., Kim D. K., Kim J. W., Park J. O., Kim S., Kim S. H., Desiderio D. M., Kim Y. K., Kim K. P., Gho Y. S. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics. (2009);9:5425–5436. doi: 10.1002/pmic.200900338. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

