Label-free photoacoustic nanoscopy
- PMID: 25104412
- PMCID: PMC4125341
- DOI: 10.1117/1.JBO.19.8.086006
Label-free photoacoustic nanoscopy
Abstract
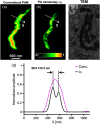
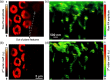
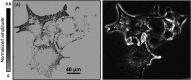
Super-resolution microscopy techniques - capable of overcoming the diffraction limit of light - have opened new opportunities to explore subcellular structures and dynamics not resolvable in conventional far-field microscopy. However, relying on staining with exogenous fluorescent markers, these techniques can sometimes introduce undesired artifacts to the image, mainly due to large tagging agent sizes and insufficient or variable labeling densities. By contrast, the use of endogenous pigments allows imaging of the intrinsic structures of biological samples with unaltered molecular constituents. Here, we report label-free photoacoustic (PA) nanoscopy, which is exquisitely sensitive to optical absorption, with an 88 nm resolution. At each scanning position, multiple PA signals are successively excited with increasing laser pulse energy. Because of optical saturation or nonlinear thermal expansion, the PA amplitude depends on the nonlinear incident optical fluence. The high-order dependence, quantified by polynomial fitting, provides super-resolution imaging with optical sectioning. PA nanoscopy is capable of super-resolution imaging of either fluorescent or nonfluorescent molecules.
Figures




References
-
- Hell S. W., Kroug M., “Ground-state-depletion fluorescence microscopy—a concept for breaking the diffraction resolution limit,” Appl. Phys. B 60(5), 495–497 (1995).APBOEM10.1007/BF01081333 - DOI

