Sources of DNA double-strand breaks and models of recombinational DNA repair
- PMID: 25104768
- PMCID: PMC4142968
- DOI: 10.1101/cshperspect.a016428
Sources of DNA double-strand breaks and models of recombinational DNA repair
Abstract
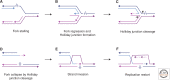
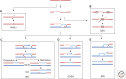
DNA is subject to many endogenous and exogenous insults that impair DNA replication and proper chromosome segregation. DNA double-strand breaks (DSBs) are one of the most toxic of these lesions and must be repaired to preserve chromosomal integrity. Eukaryotes are equipped with several different, but related, repair mechanisms involving homologous recombination, including single-strand annealing, gene conversion, and break-induced replication. In this review, we highlight the chief sources of DSBs and crucial requirements for each of these repair processes, as well as the methods to identify and study intermediate steps in DSB repair by homologous recombination.
Copyright © 2014 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
