Development and matching of binocular orientation preference in mouse V1
- PMID: 25104927
- PMCID: PMC4109519
- DOI: 10.3389/fnsys.2014.00128
Development and matching of binocular orientation preference in mouse V1
Abstract
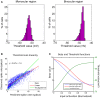
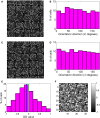
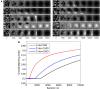
Eye-specific thalamic inputs converge in the primary visual cortex (V1) and form the basis of binocular vision. For normal binocular perceptions, such as depth and stereopsis, binocularly matched orientation preference between the two eyes is required. A critical period of binocular matching of orientation preference in mice during normal development is reported in literature. Using a reaction diffusion model we present the development of RF and orientation selectivity in mouse V1 and investigate the binocular orientation preference matching during the critical period. At the onset of the critical period the preferred orientations of the modeled cells are mostly mismatched in the two eyes and the mismatch decreases and reaches levels reported in juvenile mouse by the end of the critical period. At the end of critical period 39% of cells in binocular zone in our model cortex is orientation selective. In literature around 40% cortical cells are reported as orientation selective in mouse V1. The starting and the closing time for critical period determine the orientation preference alignment between the two eyes and orientation tuning in cortical cells. The absence of near neighbor interaction among cortical cells during the development of thalamo-cortical wiring causes a salt and pepper organization in the orientation preference map in mice. It also results in much lower % of orientation selective cells in mice as compared to ferrets and cats having organized orientation maps with pinwheels.
Keywords: critical period for orientation matching; mouse V1; orientation map; orientation selectivity; receptive field alignment.
Figures







References
-
- Bishop P. O., Henry G. H. (1972). Striate neurones: receptive field concepts. Invest. Ophthalmol. 11, 346–354 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

