HCV RNA viral load is independent from CD4 cell count and plasma HIV RNA viral load in immunocompetent HIV-HCV co-infected patients: a 3-years follow-up study
- PMID: 25104966
- PMCID: PMC4124775
- DOI: 10.1186/1742-6405-11-21
HCV RNA viral load is independent from CD4 cell count and plasma HIV RNA viral load in immunocompetent HIV-HCV co-infected patients: a 3-years follow-up study
Abstract
Background: HCV RNA viral load is an important predictor of sustained virological response and, recently, a significant correlation with liver fibrosis was described. We investigated on possible influence of clinical and viro-immunological variables on HCV viral load in HIV-HCV co-infected patients over a study time of three years (2009-2012).
Methods: We retrospectively enrolled 98 adult patients with a diagnosis of chronic HIV infection in 2009, a diagnosis of chronic HCV infection with a detectable plasma HCV RNA in 2009 and 2012, HCV therapy-naïve or with failed and stopped antiviral treatment before June 2008. The following variables were recorded: age, gender, HCV genotype, IL28B rs12979860 CC genotype, HCV treatment status, advanced liver fibrosis diagnosis, antiretroviral therapy, CD4+ cell count, HCV viral load, HIV RNA (plasma HIV-1 RNA levels were measured from blood samples every three months at least). The correlation was established using linear regression analysis, analysis of variance and Fisher's exact test. Comparisons between groups were performed using Fisher's exact test, the independent samples t-test and the t-test for paired data, as appropriate, for continuous variables. A mixed mode (ME) maximum likelihood linear regression model was constructed to evaluate the dependence of HCV viral load.
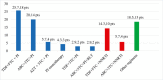
Results: HCV RNA levels did not change significantly from 2009 to 2012 (from 3924650 ± 5320177 IU/ml to 3085128 ± 3372347 IU/ml, p = 0.13); the CD4+ count increased significantly (from a mean of 576 to a mean of 654, p = 0.003). Using linear regression, a positive correlation was observed for HCV load and genotype 1 (p = 0.002), nonresponder status (p = 0.04) and with interleukin 28B CC allele (p = 0.05). Other studied covariates failed to reach a significant correlation.
Conclusions: The HCV RNA load, a known pretreatment predictor of response to antiviral therapy, was independent of the two main parameters of HIV disease, plasma HIV RNA and CD4 cell count, over an observation time of 3 years in patients with recovered or spontaneously maintained immunocompetence.
Keywords: Follow-up; HCV RNA; HCV genotype 1; HIV RNA; Interleukin 28B CC allele.
Figures

References
-
- Andreoni M, Giacometti A, Maida I, Meraviglia P, Ripamonti D, Sarmati L. HIV-HCV co-infection: epidemiology, pathogenesis and therapeutic implications. Eur Rev Med Pharmacol Sci. 2012;16:1473–1483. - PubMed
-
- Berenguer J, Rodríguez E, Miralles P, Von Wichmann MA, López-Aldeguer J, Mallolas J, Galindo MJ, Van Den Eynde E, Téllez MJ, Quereda C, Jou A, Sanz J, Barros C, Santos I, Pulido F, Guardiola JM, Ortega E, Rubio R, Jusdado JJ, Montes ML, Gaspar G, Esteban H, Bellón JM, González-García J. Sustained virological response to interferon plus ribavirin reduces non–liver-related mortality in patients coinfected with HIV and Hepatitis C virus. Clin Infect Dis. 2012;55:728–736. doi: 10.1093/cid/cis500. - DOI - PubMed
-
- Basso M, Parisi SG, Mengoli C, Gentilini V, Menegotto N, Monticelli J, Nicolè S, Cruciani M, Palù G. Sustained virological response and baseline predictors in HIV-HCV coinfected patients retreated with pegylated interferon and ribavirin after failing a previous interferon-based therapy: systematic review and meta-analysis. HIV Clin Trials. 2013;14:127–139. doi: 10.1310/hct1404-127. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

