Regulation of autophagy by α1-antitrypsin: "a foe of a foe is a friend"
- PMID: 25105300
- PMCID: PMC4212011
- DOI: 10.2119/molmed.2014.00054
Regulation of autophagy by α1-antitrypsin: "a foe of a foe is a friend"
Abstract
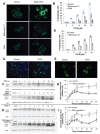
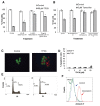
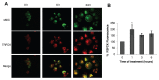
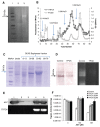
Autophagy is involved in both the cell protective and the cell death process but its mechanism is largely unknown. The present work unravels a novel intracellular mechanism by which the serpin α1-antitrypsin (AAT) acts as a novel negative regulator of autophagic cell death. For the first time, the role of intracellularly synthesized AAT, other than in liver cells, is demonstrated. Autophagic cell death was induced by N-α-tosyl-L-phenylalanine chloromethyl ketone (TPCK) and tamoxifen. By utilizing a fluorescently tagged TPCK analog, AAT was "fished out" (pulled out) as a TPCK intracellular protein target. The interaction was further verified by competition binding experiments. Both inducers caused downregulation of AAT expression associated with activation of trypsin-like proteases. Furthermore, silencing AAT by siRNA induced autophagic cell death. Moreover, AAT administration to cultured cells prevented autophagic cell death. This new mechanism could have implications in the treatment of diseases by the regulation of AAT levels in which autophagy has a detrimental function. Furthermore, the results imply that the high synthesis of endogenous AAT by cancer cells could provide a novel resistance mechanism of cancer against autophagic cell death.
Figures


References
-
- Codogno P, Meijer AJ. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ. 2005;12(Suppl 2):1509–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

