FCGR2C polymorphisms associate with HIV-1 vaccine protection in RV144 trial
- PMID: 25105367
- PMCID: PMC4151214
- DOI: 10.1172/JCI75539
FCGR2C polymorphisms associate with HIV-1 vaccine protection in RV144 trial
Abstract
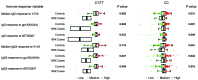
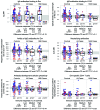
The phase III RV144 HIV-1 vaccine trial estimated vaccine efficacy (VE) to be 31.2%. This trial demonstrated that the presence of HIV-1-specific IgG-binding Abs to envelope (Env) V1V2 inversely correlated with infection risk, while the presence of Env-specific plasma IgA Abs directly correlated with risk of HIV-1 infection. Moreover, Ab-dependent cellular cytotoxicity responses inversely correlated with risk of infection in vaccine recipients with low IgA; therefore, we hypothesized that vaccine-induced Fc receptor-mediated (FcR-mediated) Ab function is indicative of vaccine protection. We sequenced exons and surrounding areas of FcR-encoding genes and found one FCGR2C tag SNP (rs114945036) that associated with VE against HIV-1 subtype CRF01_AE, with lysine at position 169 (169K) in the V2 loop (CRF01_AE 169K). Individuals carrying CC in this SNP had an estimated VE of 15%, while individuals carrying CT or TT exhibited a VE of 91%. Furthermore, the rs114945036 SNP was highly associated with 3 other FCGR2C SNPs (rs138747765, rs78603008, and rs373013207). Env-specific IgG and IgG3 Abs, IgG avidity, and neutralizing Abs inversely correlated with CRF01_AE 169K HIV-1 infection risk in the CT- or TT-carrying vaccine recipients only. These data suggest a potent role of Fc-γ receptors and Fc-mediated Ab function in conferring protection from transmission risk in the RV144 VE trial.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- UM1AI-068618/AI/NIAID NIH HHS/United States
- U01 AI067854/AI/NIAID NIH HHS/United States
- P30 CA015704/CA/NCI NIH HHS/United States
- R01 AI102718/AI/NIAID NIH HHS/United States
- R37AI054165/AI/NIAID NIH HHS/United States
- R37 AI054165/AI/NIAID NIH HHS/United States
- R01 AI098485/AI/NIAID NIH HHS/United States
- UM1 AI068635/AI/NIAID NIH HHS/United States
- P01 AI100151/AI/NIAID NIH HHS/United States
- AI067854/AI/NIAID NIH HHS/United States
- UM1 AI068618/AI/NIAID NIH HHS/United States
- R01 HL114901/HL/NHLBI NIH HHS/United States
- U19 AI067854/AI/NIAID NIH HHS/United States
- Y1-AI-2642-12/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

