Splice-correcting oligonucleotides restore BTK function in X-linked agammaglobulinemia model
- PMID: 25105368
- PMCID: PMC4151202
- DOI: 10.1172/JCI76175
Splice-correcting oligonucleotides restore BTK function in X-linked agammaglobulinemia model
Abstract
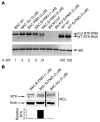
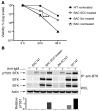
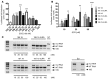
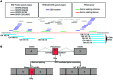
X-linked agammaglobulinemia (XLA) is an inherited immunodeficiency that results from mutations within the gene encoding Bruton's tyrosine kinase (BTK). Many XLA-associated mutations affect splicing of BTK pre-mRNA and severely impair B cell development. Here, we assessed the potential of antisense, splice-correcting oligonucleotides (SCOs) targeting mutated BTK transcripts for treating XLA. Both the SCO structural design and chemical properties were optimized using 2'-O-methyl, locked nucleic acid, or phosphorodiamidate morpholino backbones. In order to have access to an animal model of XLA, we engineered a transgenic mouse that harbors a BAC with an authentic, mutated, splice-defective human BTK gene. BTK transgenic mice were bred onto a Btk knockout background to avoid interference of the orthologous mouse protein. Using this model, we determined that BTK-specific SCOs are able to correct aberrantly spliced BTK in B lymphocytes, including pro-B cells. Correction of BTK mRNA restored expression of functional protein, as shown both by enhanced lymphocyte survival and reestablished BTK activation upon B cell receptor stimulation. Furthermore, SCO treatment corrected splicing and restored BTK expression in primary cells from patients with XLA. Together, our data demonstrate that SCOs can restore BTK function and that BTK-targeting SCOs have potential as personalized medicine in patients with XLA.
Figures





References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

