Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function
- PMID: 25105578
- PMCID: PMC4212493
- DOI: 10.1016/j.stem.2014.07.012
Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function
Abstract
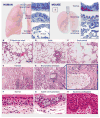
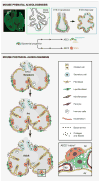
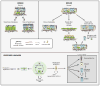
Respiratory disease is the third leading cause of death in the industrialized world. Consequently, the trachea, lungs, and cardiopulmonary vasculature have been the focus of extensive investigations. Recent studies have provided new information about the mechanisms driving lung development and differentiation. However, there is still much to learn about the ability of the adult respiratory system to undergo repair and to replace cells lost in response to injury and disease. This Review highlights the multiple stem/progenitor populations in different regions of the adult lung, the plasticity of their behavior in injury models, and molecular pathways that support homeostasis and repair.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


References
-
- Aumiller V, Balsara N, Wilhelm J, Gunther A, Konigshoff M. WNT/beta-catenin signaling induces IL-1beta expression by alveolar epithelial cells in pulmonary fibrosis. American Journal of Respiratory Cell and Molecular Biology. 2013;49:96–104. - PubMed
Publication types
MeSH terms
Grants and funding
- T32 HL007749/HL/NHLBI NIH HHS/United States
- U01 HL111016/HL/NHLBI NIH HHS/United States
- U01 HL111146/HL/NHLBI NIH HHS/United States
- T32 GM086287/GM/NIGMS NIH HHS/United States
- U01 HL110942/HL/NHLBI NIH HHS/United States
- K08 HL119553/HL/NHLBI NIH HHS/United States
- U19 AI077439/AI/NIAID NIH HHS/United States
- L40 HL115656/HL/NHLBI NIH HHS/United States
- R01 HL064632/HL/NHLBI NIH HHS/United States
- U01 HL110964/HL/NHLBI NIH HHS/United States
- K08 HL122521/HL/NHLBI NIH HHS/United States
- R01 HL071589/HL/NHLBI NIH HHS/United States
- R01 HL087825/HL/NHLBI NIH HHS/United States
- P01 HL024136/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

