Vitamin A and feeding statuses modulate the insulin-regulated gene expression in Zucker lean and fatty primary rat hepatocytes
- PMID: 25105869
- PMCID: PMC4126667
- DOI: 10.1371/journal.pone.0100868
Vitamin A and feeding statuses modulate the insulin-regulated gene expression in Zucker lean and fatty primary rat hepatocytes
Abstract
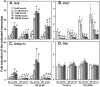
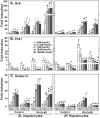
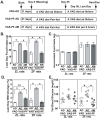
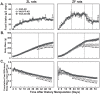
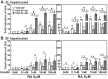
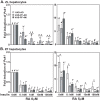
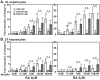
Unattended hepatic insulin resistance predisposes individuals to dyslipidemia, type 2 diabetes and many other metabolic complications. The mechanism of hepatic insulin resistance at the gene expression level remains unrevealed. To examine the effects of vitamin A (VA), total energy intake and feeding conditions on the insulin-regulated gene expression in primary hepatocytes of Zucker lean (ZL) and fatty (ZF) rats, we analyze the expression levels of hepatic model genes in response to the treatments of insulin and retinoic acid (RA). We report that the insulin- and RA-regulated glucokinase, sterol regulatory element-binding protein-1c and cytosolic form of phosphoenolpyruvate carboxykinase expressions are impaired in hepatocytes of ZF rats fed chow or a VA sufficient (VAS) diet ad libitum. The impairments are partially corrected when ZF rats are fed a VA deficient (VAD) diet ad libitum or pair-fed a VAS diet to the intake of their VAD counterparts in non-fasting conditions. Interestingly in the pair-fed ZL and ZF rats, transient overeating on the last day of pair-feeding regimen changes the expression levels of some VA catabolic genes, and impairs the insulin- and RA-regulated gene expression in hepatocytes. These results demonstrate that VA and feeding statuses modulate the hepatic insulin sensitivity at the gene expression level.
Conflict of interest statement
Figures

References
-
- O'Brien RM, Granner DK (1996) Regulation of gene expression by insulin. Physiol Rev 76: 1109–1161. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

