Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials
- PMID: 25106063
- PMCID: PMC4441266
- DOI: 10.1016/S0140-6736(14)60584-5
Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials
Abstract
Background: Alteplase is effective for treatment of acute ischaemic stroke but debate continues about its use after longer times since stroke onset, in older patients, and among patients who have had the least or most severe strokes. We assessed the role of these factors in affecting good stroke outcome in patients given alteplase.
Methods: We did a pre-specified meta-analysis of individual patient data from 6756 patients in nine randomised trials comparing alteplase with placebo or open control. We included all completed randomised phase 3 trials of intravenous alteplase for treatment of acute ischaemic stroke for which data were available. Retrospective checks confirmed that no eligible trials had been omitted. We defined a good stroke outcome as no significant disability at 3-6 months, defined by a modified Rankin Score of 0 or 1. Additional outcomes included symptomatic intracranial haemorrhage (defined by type 2 parenchymal haemorrhage within 7 days and, separately, by the SITS-MOST definition of parenchymal type 2 haemorrhage within 36 h), fatal intracranial haemorrhage within 7 days, and 90-day mortality.
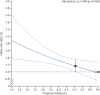
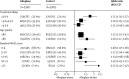
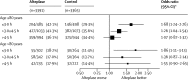
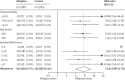
Findings: Alteplase increased the odds of a good stroke outcome, with earlier treatment associated with bigger proportional benefit. Treatment within 3·0 h resulted in a good outcome for 259 (32·9%) of 787 patients who received alteplase versus 176 (23·1%) of 762 who received control (OR 1·75, 95% CI 1·35-2·27); delay of greater than 3·0 h, up to 4·5 h, resulted in good outcome for 485 (35·3%) of 1375 versus 432 (30·1%) of 1437 (OR 1·26, 95% CI 1·05-1·51); and delay of more than 4·5 h resulted in good outcome for 401 (32·6%) of 1229 versus 357 (30·6%) of 1166 (OR 1·15, 95% CI 0·95-1·40). Proportional treatment benefits were similar irrespective of age or stroke severity. Alteplase significantly increased the odds of symptomatic intracranial haemorrhage (type 2 parenchymal haemorrhage definition 231 [6·8%] of 3391 vs 44 [1·3%] of 3365, OR 5·55, 95% CI 4·01-7·70, p<0·0001; SITS-MOST definition 124 [3·7%] vs 19 [0·6%], OR 6·67, 95% CI 4·11-10·84, p<0·0001) and of fatal intracranial haemorrhage within 7 days (91 [2·7%] vs 13 [0·4%]; OR 7·14, 95% CI 3·98-12·79, p<0·0001). The relative increase in fatal intracranial haemorrhage from alteplase was similar irrespective of treatment delay, age, or stroke severity, but the absolute excess risk attributable to alteplase was bigger among patients who had more severe strokes. There was no excess in other early causes of death and no significant effect on later causes of death. Consequently, mortality at 90 days was 608 (17·9%) in the alteplase group versus 556 (16·5%) in the control group (hazard ratio 1·11, 95% CI 0·99-1·25, p=0·07). Taken together, therefore, despite an average absolute increased risk of early death from intracranial haemorrhage of about 2%, by 3-6 months this risk was offset by an average absolute increase in disability-free survival of about 10% for patients treated within 3·0 h and about 5% for patients treated after 3·0 h, up to 4·5 h.
Interpretation: Irrespective of age or stroke severity, and despite an increased risk of fatal intracranial haemorrhage during the first few days after treatment, alteplase significantly improves the overall odds of a good stroke outcome when delivered within 4·5 h of stroke onset, with earlier treatment associated with bigger proportional benefits.
Funding: UK Medical Research Council, British Heart Foundation, University of Glasgow, University of Edinburgh.
Copyright © 2014 Emberson et al. Open Access article distributed under the terms of CC BY. Published by Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Alteplase in acute ischaemic stroke: the need for speed.Lancet. 2014 Nov 29;384(9958):1904-6. doi: 10.1016/S0140-6736(14)60662-0. Epub 2014 Aug 5. Lancet. 2014. PMID: 25106064 No abstract available.
-
Pooled RCTs: alteplase within 4.5 hours of ischemic stroke improves the likelihood of good outcome.Ann Intern Med. 2015 Jan 20;162(2):JC3. doi: 10.7326/ACPJC-2015-162-2-003. Ann Intern Med. 2015. PMID: 25599363 No abstract available.
-
Timing of thrombolysis for acute ischaemic stroke: the earlier the treatment the better the outcome, irrespective of age or stroke severity.Evid Based Med. 2015 Jun;20(3):108. doi: 10.1136/ebmed-2014-110155. Epub 2015 Apr 8. Evid Based Med. 2015. PMID: 25854628 No abstract available.
-
Thrombolysis for stroke: clinical judgment at its apogee.Lancet. 2015 Apr 11;385(9976):1366. doi: 10.1016/S0140-6736(15)60702-4. Lancet. 2015. PMID: 25890403 No abstract available.
-
Thrombolysis in acute stroke.Lancet. 2015 Apr 11;385(9976):1394-5. doi: 10.1016/S0140-6736(15)60715-2. Lancet. 2015. PMID: 25890416 No abstract available.
-
Thrombolysis in acute stroke.Lancet. 2015 Apr 11;385(9976):1394. doi: 10.1016/S0140-6736(15)60714-0. Lancet. 2015. PMID: 25890417 No abstract available.
-
Thrombolysis in acute stroke.Lancet. 2015 Apr 11;385(9976):1395. doi: 10.1016/S0140-6736(15)60716-4. Lancet. 2015. PMID: 25890418 No abstract available.
-
Thrombolysis in acute stroke.Lancet. 2015 Apr 11;385(9976):1395-6. doi: 10.1016/S0140-6736(15)60717-6. Lancet. 2015. PMID: 25890419 No abstract available.
-
Thrombolysis in acute stroke--authors' reply.Lancet. 2015 Apr 11;385(9976):1396. doi: 10.1016/S0140-6736(15)60718-8. Lancet. 2015. PMID: 25890422 No abstract available.
-
Ateplase for ischaemic stroke: increased risk of intracranial haemorrhage is balanced by improved stroke outcomes, particularly if treated within 3-4.5 h of onset.Evid Based Nurs. 2015 Oct;18(4):116. doi: 10.1136/eb-2015-102056. Epub 2015 Apr 23. Evid Based Nurs. 2015. PMID: 25908693 No abstract available.
References
-
- Hacke W, Donnan G, Fieschi C, the ATLANTIS Trials Investigators. the ECASS Trials Investigators. the NINDS rt-PA Study Group Investigators Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. - PubMed
-
- Lees KR, Bluhmki E, von Kummer R, for the ECASS. ATLANTIS. NINDS and EPITHET rt-PA Study Group Investigators Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. - PubMed
-
- European Stroke Organisation (ESO) Executive Committee. the ESO Writing Committee Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25:457–507. - PubMed
-
- European Stroke Organisation guidelines for stroke management: Update January 2009. Available from: http://www.eso-stroke.org/eso-stroke/education/education... (accessed March 3, 2014).
-
- Minematsu K, Toyoda K, Hirano T. Guidelines for the intravenous application of recombinant tissue-type plasminogen activator (alteplase), the second edition, October 2012: a guideline from the Japan Stroke Society. J Stroke Cerebrovasc. 2013;22:571–600. - PubMed

