Hydrophobic gating in ion channels
- PMID: 25106689
- PMCID: PMC4817205
- DOI: 10.1016/j.jmb.2014.07.030
Hydrophobic gating in ion channels
Abstract
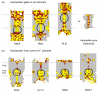
Biological ion channels are nanoscale transmembrane pores. When water and ions are enclosed within the narrow confines of a sub-nanometer hydrophobic pore, they exhibit behavior not evident from macroscopic descriptions. At this nanoscopic level, the unfavorable interaction between the lining of a hydrophobic pore and water may lead to stochastic liquid-vapor transitions. These transient vapor states are "dewetted", i.e. effectively devoid of water molecules within all or part of the pore, thus leading to an energetic barrier to ion conduction. This process, termed "hydrophobic gating", was first observed in molecular dynamics simulations of model nanopores, where the principles underlying hydrophobic gating (i.e., changes in diameter, polarity, or transmembrane voltage) have now been extensively validated. Computational, structural, and functional studies now indicate that biological ion channels may also exploit hydrophobic gating to regulate ion flow within their pores. Here we review the evidence for this process and propose that this unusual behavior of water represents an increasingly important element in understanding the relationship between ion channel structure and function.
Keywords: K2P channel; hydrophobic gating; ion channel; nanopore; potassium channel.
Copyright © 2014. Published by Elsevier Ltd.
Figures

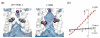
References
-
- Hummer G, Rasaiah JC, Noworyta JP. Water conduction through the hydrophobic channel of a carbon nanotube. Nature. 2001;414:188–90. - PubMed
-
- Beckstein O, Biggin PC, Sansom MSP. A hydrophobic gating mechanism for nanopores. J Phys Chem B. 2001;105:12902–12905.
-
- Allen R, Hansen JP, Melchionna S. Molecular dynamics investigation of water permeation through nanopores. Journal of Chemical Physics. 2003;119:3905–3919.
-
- Beckstein O, Tai K, Sansom MSP. Not Ions Alone: Barriers to Ion Permeation in Nanopores and Channels. Journal of the American Chemical Society. 2004;126:14694–14695. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/L002558/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/B/16011/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BEP17032/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- B19456/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/H000267/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

