Fractures in geriatric mice show decreased callus expansion and bone volume
- PMID: 25106797
- PMCID: PMC4182401
- DOI: 10.1007/s11999-014-3829-x
Fractures in geriatric mice show decreased callus expansion and bone volume
Abstract
Background: Poor fracture healing in geriatric populations is a significant source of morbidity, mortality, and cost to individuals and society; however, a fundamental biologic understanding of age-dependent healing remains elusive. The development of an aged-based fracture model system would allow for a mechanistic understanding that could guide future biologic treatments.
Questions/purposes: Using a small animal model of long-bone fracture healing based on chronologic age, we asked how aging affected (1) the amount, density, and proportion of bone formed during healing; (2) the amount of cartilage produced and the progression to bone during healing; (3) the callus structure and timing of the fracture healing; and (4) the behavior of progenitor cells relative to the observed deficiencies of geriatric fracture healing.
Methods: Transverse, traumatic tibial diaphyseal fractures were created in 5-month-old (n=104; young adult) and 25-month-old (n=107; which we defined as geriatric, and are approximately equivalent to 70-85 year-old humans) C57BL/6 mice. Fracture calluses were harvested at seven times from 0 to 40 days postfracture for micro-CT analysis (total volume, bone volume, bone volume fraction, connectivity density, structure model index, trabecular number, trabecular thickness, trabecular spacing, total mineral content, bone mineral content, tissue mineral density, bone mineral density, degree of anisotropy, and polar moment of inertia), histomorphometry (total callus area, cartilage area, percent of cartilage, hypertrophic cartilage area, percent of hypertrophic cartilage area, bone and osteoid area, percent of bone and osteoid area), and gene expression quantification (fold change).
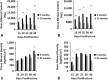
Results: The geriatric mice produced a less robust healing response characterized by a pronounced decrease in callus amount (mean total volume at 20 days postfracture, 30.08±11.53 mm3 versus 43.19±18.39 mm3; p=0.009), density (mean bone mineral density at 20 days postfracture, 171.14±64.20 mg hydroxyapatite [HA]/cm3 versus 210.79±37.60 mg HA/cm3; p=0.016), and less total cartilage (mean cartilage area at 10 days postfracture, 101,279±46,755 square pixels versus 302,167±137,806 square pixels; p=0.013) and bone content (mean bone volume at 20 days postfracture, 11.68±3.18 mm3 versus 22.34±10.59 mm3; p<0.001) compared with the young adult mice. However, the amount of cartilage and bone relative to the total callus size was similar between the adult and geriatric mice (mean bone volume fraction at 25 days postfracture, 0.48±0.10 versus 0.50±0.13; p=0.793), and the relative expression of chondrogenic (mean fold change in SOX9 at 10 days postfracture, 135+25 versus 90±52; p=0.221) and osteogenic genes (mean fold change in osterix at 20 days postfracture, 22.2±5.3 versus 18.7±5.2; p=0.324) was similar. Analysis of mesenchymal cell proliferation in the geriatric mice relative to adult mice showed a decrease in proliferation (mean percent of undifferentiated mesenchymal cells staining proliferating cell nuclear antigen [PCNA] positive at 10 days postfracture, 25%±6.8% versus 42%±14.5%; p=0.047).
Conclusions: Our findings suggest that the molecular program of fracture healing is intact in geriatric mice, as it is in geriatric humans, but callus expansion is reduced in magnitude.
Clinical relevance: Our study showed altered healing capacity in a relevant animal model of geriatric fracture healing. The understanding that callus expansion and bone volume are decreased with aging can help guide the development of targeted therapeutics for these difficult to heal fractures.
Figures
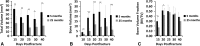



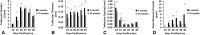
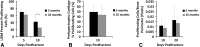

Comment in
-
CORR Insights®: fractures in geriatric mice show decreased callus expansion and bone volume.Clin Orthop Relat Res. 2014 Nov;472(11):3533-5. doi: 10.1007/s11999-014-3892-3. Epub 2014 Aug 21. Clin Orthop Relat Res. 2014. PMID: 25141847 Free PMC article. No abstract available.
References
-
- Arias E. United States Life Tables, 2006. National Vital Statistics Reports. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr58/nvsr58_21.pdf. Accessed January 30, 2013. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

