Overexpression of Galnt3 in chondrocytes resulted in dwarfism due to the increase of mucin-type O-glycans and reduction of glycosaminoglycans
- PMID: 25107907
- PMCID: PMC4176229
- DOI: 10.1074/jbc.M114.555987
Overexpression of Galnt3 in chondrocytes resulted in dwarfism due to the increase of mucin-type O-glycans and reduction of glycosaminoglycans
Abstract
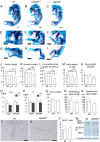
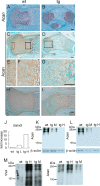
Galnt3, UDP-N-acetyl-α-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 3, transfers N-acetyl-D-galactosamine to serine and threonine residues, initiating mucin type O-glycosylation of proteins. We searched the target genes of Runx2, which is an essential transcription factor for chondrocyte maturation, in chondrocytes and found that Galnt3 expression was up-regulated by Runx2 and severely reduced in Runx2(-/-) cartilaginous skeletons. To investigate the function of Galnt3 in chondrocytes, we generated Galnt3(-/-) mice and chondrocyte-specific Galnt3 transgenic mice under the control of the Col2a1 promoter-enhancer. Galnt3(-/-) mice showed a delay in endochondral ossification and shortened limbs at embryonic day 16.5, suggesting that Galnt3 is involved in chondrocyte maturation. Galnt3 transgenic mice presented dwarfism, the chondrocyte maturation was retarded, the cell cycle in chondrocytes was accelerated, premature chondrocyte apoptosis occurred, and the growth plates were disorganized. The binding of Vicia villosa agglutinin, which recognizes the Tn antigen (GalNAc-O-Ser/Thr), was drastically increased in chondrocytes, and aggrecan (Acan) was highly enriched with Tn antigen. However, safranin O staining, which recognizes glycosaminoglycans (GAGs), and Acan were severely reduced. Chondroitin sulfate was reduced in amount, but the elongation of chondroitin sulfate chains had not been severely disturbed in the isolated GAGs. These findings indicate that overexpression of Galnt3 in chondrocytes caused dwarfism due to the increase of mucin-type O-glycans and the reduction of GAGs, probably through competition with xylosyltransferases, which initiate GAG chains by attaching O-linked xylose to serine residues, suggesting a negative effect of Galnt family proteins on Acan deposition in addition to the positive effect of Galnt3 on chondrocyte maturation.
Keywords: Aggrecan; Chondrocyte; Galnt3; Glycosaminoglycan; Glycosyltransferase; Mucin; Mucin-type O-Glycan; Proteoglycan.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures








References
-
- Zhao Q., Eberspaecher H., Lefebvre V., De Crombrugghe B. (1997) Parallel expression of Sox9 and Col2a1 in cells undergoing chondrogenesis. Dev. Dyn. 209, 377–386 - PubMed
-
- Gentili C., Cancedda R. (2009) Cartilage and bone extracellular matrix. Curr. Pharm. Des. 15, 1334–1348 - PubMed
-
- Varki A., Cummings R. D., Esko J. D., Freeze H. H., Stanley P., Bertozzi C. R., Hart G. W., Etzler M. E. (eds) (2009) Essentials of Glycobiology, pp. 229–248, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

