Membrane dynamics at the nuclear exchange junction during early mating (one to four hours) in the ciliate Tetrahymena thermophila
- PMID: 25107923
- PMCID: PMC4311919
- DOI: 10.1128/EC.00164-14
Membrane dynamics at the nuclear exchange junction during early mating (one to four hours) in the ciliate Tetrahymena thermophila
Abstract
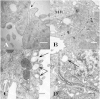
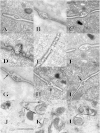
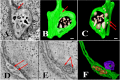
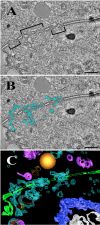
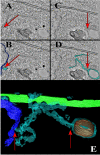
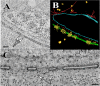
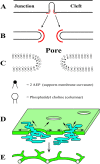
Using serial-section transmission electron microscopy and three-dimensional (3D) electron tomography, we characterized membrane dynamics that accompany the construction of a nuclear exchange junction between mating cells in the ciliate Tetrahymena thermophila. Our methods revealed a number of previously unknown features. (i) Membrane fusion is initiated by the extension of hundreds of 50-nm-diameter protrusions from the plasma membrane. These protrusions extend from both mating cells across the intercellular space to fuse with membrane of the mating partner. (ii) During this process, small membrane-bound vesicles or tubules are shed from the plasma membrane and into the extracellular space within the junction. The resultant vesicle-filled pockets within the extracellular space are referred to as junction lumens. (iii) As junction lumens fill with extracellular microvesicles and swell, the plasma membrane limiting these swellings undergoes another deformation, pinching off vesicle-filled vacuoles into the cytoplasm (reclamation). (iv) These structures (resembling multivesicular bodies) seem to associate with autophagosomes abundant near the exchange junction. We propose a model characterizing the membrane-remodeling events that establish cytoplasmic continuity between mating Tetrahymena cells. We also discuss the possible role of nonvesicular lipid transport in conditioning the exchange junction lipid environment. Finally, we raise the possibility of an intercellular signaling mechanism involving microvesicle shedding and uptake.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
Restoration of cellular integrity following "ballistic" pronuclear exchange during Tetrahymena conjugation.Dev Biol. 2018 Dec 1;444(1):33-40. doi: 10.1016/j.ydbio.2018.09.019. Epub 2018 Sep 27. Dev Biol. 2018. PMID: 30268714
-
The Role of Membrane-Bound Extracellular Vesicles During Co-Stimulation and Conjugation in the Ciliate Tetrahymena thermophila.Microorganisms. 2025 Apr 1;13(4):803. doi: 10.3390/microorganisms13040803. Microorganisms. 2025. PMID: 40284639 Free PMC article.
-
Close cooperation between Semi1 and Semi2 proteins is essential for pronuclear positioning in Tetrahymena thermophila.Mol Biol Cell. 2025 Mar 1;36(3):ar23. doi: 10.1091/mbc.E24-11-0503. Epub 2025 Jan 9. Mol Biol Cell. 2025. PMID: 39785685 Free PMC article.
-
An evolutionary balance: conservation vs innovation in ciliate membrane trafficking.Traffic. 2017 Jan;18(1):18-28. doi: 10.1111/tra.12450. Epub 2016 Oct 27. Traffic. 2017. PMID: 27696651 Free PMC article. Review.
-
Out with a bang! Tetrahymena as a model system to study secretory granule biogenesis.Traffic. 2004 Feb;5(2):63-8. doi: 10.1046/j.1600-0854.2003.00155.x. Traffic. 2004. PMID: 14690495 Review.
Cited by
-
Snf2 Proteins Are Required to Generate Gamete Pronuclei in Tetrahymena thermophila.Microorganisms. 2022 Dec 7;10(12):2426. doi: 10.3390/microorganisms10122426. Microorganisms. 2022. PMID: 36557679 Free PMC article.
-
Unexpected organellar locations of ESCRT machinery in Giardia intestinalis and complex evolutionary dynamics spanning the transition to parasitism in the lineage Fornicata.BMC Biol. 2021 Aug 27;19(1):167. doi: 10.1186/s12915-021-01077-2. BMC Biol. 2021. PMID: 34446013 Free PMC article.
-
HAP2-Mediated Gamete Fusion: Lessons From the World of Unicellular Eukaryotes.Front Cell Dev Biol. 2022 Jan 7;9:807313. doi: 10.3389/fcell.2021.807313. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35071241 Free PMC article. Review.
-
The Oxymonad Genome Displays Canonical Eukaryotic Complexity in the Absence of a Mitochondrion.Mol Biol Evol. 2019 Oct 1;36(10):2292-2312. doi: 10.1093/molbev/msz147. Mol Biol Evol. 2019. PMID: 31387118 Free PMC article.
-
Novel requirements for HAP2/GCS1-mediated gamete fusion in Tetrahymena.iScience. 2024 May 28;27(6):110146. doi: 10.1016/j.isci.2024.110146. eCollection 2024 Jun 21. iScience. 2024. PMID: 38904066 Free PMC article.
References
-
- Wolfe J. 1982. The conjugation junction of Tetrahymena: its structure and development. J Morphol 172:159–178. - PubMed
-
- Orias JD, Hamilton EP, Orias E. 1983. A microtubular meshwork associated with gametic pronuclear transfer across a cell-cell junction. Science 222:181–184. - PubMed
-
- Suganuma Y, Shimode C, Yamamoto H. 1984. Conjugation in Tetrahymena: formation of a special junction area for conjugation during the co-stimulation period. J Electron Microsc (Tokyo) 33:10–18. - PubMed
-
- Cole ES. 2006. The Tetrahymena conjugation junction, p 39–62. In Baluska F, Volkmann D, Barlow PW (ed), Cell-cell channels. Springer, New York, NY.
-
- Bruns PJ, Brussard TB. 1974. Pair formation in Tetrahymena pyriformis, an inducible developmental system. J Exp Zool 188:337–344. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

