Dynamics of dual infection with Campylobacter jejuni strains in chickens reveals distinct strain-to-strain variation in infection ecology
- PMID: 25107966
- PMCID: PMC4178652
- DOI: 10.1128/AEM.01901-14
Dynamics of dual infection with Campylobacter jejuni strains in chickens reveals distinct strain-to-strain variation in infection ecology
Abstract
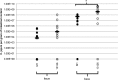
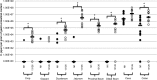
Although multiple genotypes of Campylobacter jejuni may be isolated from the same commercial broiler flock, little is known about the infection dynamics of different genotypes within individuals or their colonization sites within the gut. Single experimental infections with C. jejuni M1 (sequence type 137, clonal complex 45) and C. jejuni 13126 (sequence type 21, clonal complex 21) revealed that 13126 colonized the ceca at significantly higher levels. The dissemination and colonization sites of the two C. jejuni strains then were examined in an experimental broiler flock. Two 33-day-old broiler chickens were infected with M1 and two with 13126, and 15 birds were left unchallenged. Cloacal swabs were taken postinfection to determine the colonization and shedding of each strain. By 2 days postinfection (dpi), 8/19 birds were shedding M1 whereas none were shedding 13126. At 8 dpi, all birds were shedding both strains. At 18 dpi, liver and cecal levels of each isolate were quantified, while in 10 birds they also were quantified at nine sites throughout the gastrointestinal (GI) tract. 13126 was found throughout the GI tract, while M1 was largely restricted to the ceca and colon. The livers of 7/19 birds were culture positive for 13126 only. These data show that 13126 has a distinctly different infection biology than strain M1. It showed slower colonization of the lower GI tract but was more invasive and able to colonize at a high level throughout the GI tract. The finding that C. jejuni strains have markedly different infection ecologies within the chicken has implications for control in the poultry industry and suggests that the contamination risk of edible tissues is dependent on the isolate involved.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
Campylobacter jejuni colonization promotes the translocation of Escherichia coli to extra-intestinal organs and disturbs the short-chain fatty acids profiles in the chicken gut.Poult Sci. 2016 Oct 1;95(10):2259-65. doi: 10.3382/ps/pew151. Epub 2016 May 3. Poult Sci. 2016. PMID: 27143773
-
Heterogeneity in the Infection Biology of Campylobacter jejuni Isolates in Three Infection Models Reveals an Invasive and Virulent Phenotype in a ST21 Isolate from Poultry.PLoS One. 2015 Oct 23;10(10):e0141182. doi: 10.1371/journal.pone.0141182. eCollection 2015. PLoS One. 2015. PMID: 26496441 Free PMC article.
-
Campylobacter jejuni ST353 and ST464 cause localized gut inflammation, crypt damage, and extraintestinal spread during large- and small-scale infection in broiler chickens.Appl Environ Microbiol. 2025 Mar 19;91(3):e0161424. doi: 10.1128/aem.01614-24. Epub 2025 Feb 18. Appl Environ Microbiol. 2025. PMID: 39964091 Free PMC article.
-
Colonization factors of Campylobacter jejuni in the chicken gut.Vet Res. 2011 Jun 29;42(1):82. doi: 10.1186/1297-9716-42-82. Vet Res. 2011. PMID: 21714866 Free PMC article. Review.
-
A tolerogenic mucosal immune response leads to persistent Campylobacter jejuni colonization in the chicken gut.Crit Rev Microbiol. 2012 Feb;38(1):17-29. doi: 10.3109/1040841X.2011.615298. Epub 2011 Oct 13. Crit Rev Microbiol. 2012. PMID: 21995731 Review.
Cited by
-
Extensive characterization of Campylobacter jejuni chicken isolates to uncover genes involved in the ability to compete for gut colonization.BMC Microbiol. 2015 May 10;15:97. doi: 10.1186/s12866-015-0433-5. BMC Microbiol. 2015. PMID: 25958385 Free PMC article.
-
The transmission dynamics of Campylobacter jejuni among broilers in semi-commercial farms in Jordan.Epidemiol Infect. 2019 Jan;147:e134. doi: 10.1017/S0950268818003308. Epidemiol Infect. 2019. PMID: 30868986 Free PMC article.
-
Serotonin modulates Campylobacter jejuni physiology and invitro interaction with the gut epithelium.Poult Sci. 2021 Mar;100(3):100944. doi: 10.1016/j.psj.2020.12.041. Epub 2021 Jan 5. Poult Sci. 2021. PMID: 33652538 Free PMC article.
-
Transcriptomic analysis of caecal tissue in inbred chicken lines that exhibit heritable differences in resistance to Campylobacter jejuni.BMC Genomics. 2021 Jun 4;22(1):411. doi: 10.1186/s12864-021-07748-2. BMC Genomics. 2021. PMID: 34082718 Free PMC article.
-
Recent Advances in Screening of Anti-Campylobacter Activity in Probiotics for Use in Poultry.Front Microbiol. 2016 May 31;7:553. doi: 10.3389/fmicb.2016.00553. eCollection 2016. Front Microbiol. 2016. PMID: 27303366 Free PMC article. Review.
References
-
- EFSA. 2010. Analysis of the baseline survey on the prevalence of Campylobacter in broiler batches and of Campylobacter and Salmonella on broiler carcasses in the EU, 2008. Part A: Campylobacter and Salmonella prevalence estimates. EFSA J. 8:1503–1602. 10.2903/j.efsa.2010.1503 - DOI
-
- Young CR, Ziprin RL, Hume ME, Stanker LH. 1999. Dose response and organ invasion of day-of-hatch leghorn chicks by different isolates of Campylobacter jejuni. Avian Dis. 43:763–767 - PubMed
-
- Achen M, Morishita TY, Ley EC. 1998. Shedding and colonization of Campylobacter jejuni in broilers from day-of-hatch to slaughter age. Avian Dis. 42:732–737 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

