Physiological levels of lipoxin A4 inhibit ENaC and restore airway surface liquid height in cystic fibrosis bronchial epithelium
- PMID: 25107986
- PMCID: PMC4246599
- DOI: 10.14814/phy2.12093
Physiological levels of lipoxin A4 inhibit ENaC and restore airway surface liquid height in cystic fibrosis bronchial epithelium
Abstract
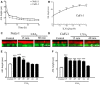
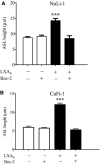
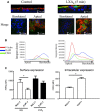
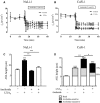
In cystic fibrosis (CF), the airway surface liquid (ASL) is depleted. We previously demonstrated that lipoxin A4 (LXA4) can modulate ASL height (ASLh) through actions on Cl(-) transport. Here, we report novel effects of lipoxin on the epithelial Na(+) channel ENaC in this response. ASL dynamics and ion transport were studied using live-cell confocal microscopy and short-circuit current measurements in CF (CuFi-1) and non-CF (NuLi-1) cell cultures. Low physiological concentrations of LXA4 in the picomolar range produced an increase in ASLh which was dependent on inhibition of an amiloride-sensitive Na(+) current and stimulation of a bumetanide-sensitive Cl(-) current. These ion transport and ASLh responses to LXA4 were blocked by Boc-2 an inhibitor of the specific LXA4 receptor ALX/FPR2. LXA4 affected the subcellular localization of its receptor and enhanced the localization of ALX/FPR2 at the apical membrane of CF cells. Our results provide evidence for a novel effect of low physiological concentrations of LXA4 to inhibit airway epithelial Na(+) absorption that results in an ASL height increase in CF airway epithelia.
Keywords: Cystic fibrosis; ENaC; lipoxin A4.
© 2014 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of the American Physiological Society and The Physiological Society.
Figures
References
-
- Blouquit‐Laye S., Chinet T. 2007. Ion and liquid transport across the bronchiolar epithelium. Respir. Physiol. Neurobiol.; 159:278-282. - PubMed
-
- Bonnans C., Mainprice B., Chanez P., Bousquet J., Urbach V. 2003. Lipoxin A4 stimulates a cytosolic Ca2+ increase in human bronchial epithelium. J. Biol. Chem.; 278:10879-10884. - PubMed
-
- Boucher R. C. 2004a. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur. Respir. J.; 23:146-158. - PubMed
-
- Boucher R. C. 2004b. Relationship of airway epithelial ion transport to chronic bronchitis. Proc. Am. Thorac. Soc.; 1:66-70. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

