New pharmacological strategies to fight enveloped viruses
- PMID: 25108320
- PMCID: PMC7112871
- DOI: 10.1016/j.tips.2014.06.004
New pharmacological strategies to fight enveloped viruses
Abstract
Enveloped viruses pose an important health threat because most of the persistent and many emerging viruses are enveloped. In particular, newly emerging viruses create a need to develop broad-spectrum antivirals, which usually are obtained by targeting host cell factors. Persistent viruses have developed efficient strategies to escape host immune control, and treatment options are limited. Targeting host cell factors essential for virus persistence, or immune-based therapies provide alternative approaches. In this review, we therefore focus on recent developments to generate antivirals targeting host cell factors or immune-based therapeutic approaches to fight infections with enveloped viruses.
Keywords: antiviral agents; emerging disease; host cell factors; immunotherapy.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
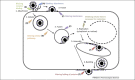
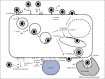
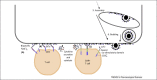
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

