Visualizing a protein quake with time-resolved X-ray scattering at a free-electron laser
- PMID: 25108686
- PMCID: PMC4149589
- DOI: 10.1038/nmeth.3067
Visualizing a protein quake with time-resolved X-ray scattering at a free-electron laser
Abstract
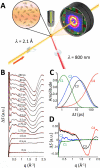
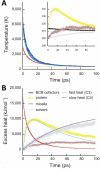
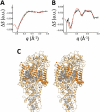
We describe a method to measure ultrafast protein structural changes using time-resolved wide-angle X-ray scattering at an X-ray free-electron laser. We demonstrated this approach using multiphoton excitation of the Blastochloris viridis photosynthetic reaction center, observing an ultrafast global conformational change that arises within picoseconds and precedes the propagation of heat through the protein. This provides direct structural evidence for a 'protein quake': the hypothesis that proteins rapidly dissipate energy through quake-like structural motions.
Figures
References
-
- Vos MH, Rappaport F, Lambry J-C, Breton J, Martin J-L. Visualization of coherent nuclear motion in a membrane protein by femtosecond spectroscopy. Nature. 1993;363:320–325.
-
- Wang H, et al. Protein dynamics control the kinetics of initial electron transfer in photosynthesis. Science. 2007;316:747–750. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

