Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up
- PMID: 25108889
- PMCID: PMC4427906
- DOI: 10.1016/S0140-6736(14)60525-0
Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up
Abstract
Background: The European Randomised study of Screening for Prostate Cancer (ERSPC) has shown significant reductions in prostate cancer mortality after 9 years and 11 years of follow-up, but screening is controversial because of adverse events such as overdiagnosis. We provide updated results of mortality from prostate cancer with follow-up to 2010, with analyses truncated at 9, 11, and 13 years.
Methods: ERSPC is a multicentre, randomised trial with a predefined centralised database, analysis plan, and core age group (55-69 years), which assesses prostate-specific antigen (PSA) testing in eight European countries. Eligible men aged 50-74 years were identified from population registries and randomly assigned by computer generated random numbers to screening or no intervention (control). Investigators were masked to group allocation. The primary outcome was prostate cancer mortality in the core age group. Analysis was by intention to treat. We did a secondary analysis that corrected for selection bias due to non-participation. Only incidence and no mortality data at 9 years' follow-up are reported for the French centres. This study is registered with Current Controlled Trials, number ISRCTN49127736.
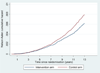
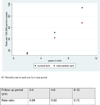
Findings: With data truncated at 13 years of follow-up, 7408 prostate cancer cases were diagnosed in the intervention group and 6107 cases in the control group. The rate ratio of prostate cancer incidence between the intervention and control groups was 1·91 (95% CI 1·83-1·99) after 9 years (1·64 [1·58-1·69] including France), 1·66 (1·60-1·73) after 11 years, and 1·57 (1·51-1·62) after 13 years. The rate ratio of prostate cancer mortality was 0·85 (0·70-1·03) after 9 years, 0·78 (0·66-0·91) after 11 years, and 0·79 (0·69-0·91) at 13 years. The absolute risk reduction of death from prostate cancer at 13 years was 0·11 per 1000 person-years or 1·28 per 1000 men randomised, which is equivalent to one prostate cancer death averted per 781 (95% CI 490-1929) men invited for screening or one per 27 (17-66) additional prostate cancer detected. After adjustment for non-participation, the rate ratio of prostate cancer mortality in men screened was 0·73 (95% CI 0·61-0·88).
Interpretation: In this update the ERSPC confirms a substantial reduction in prostate cancer mortality attributable to testing of PSA, with a substantially increased absolute effect at 13 years compared with findings after 9 and 11 years. Despite our findings, further quantification of harms and their reduction are still considered a prerequisite for the introduction of populated-based screening.
Funding: Each centre had its own funding responsibility.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflicts of interest
Dr. Lilja reports grants from National Cancer Institute grants R01CA160816; P50-CA92629 to MSKCC, New York, NY, USA, grants from David H. Koch trough the Prostate Cancer Foundation funding to the The Sidney Kimmel centre for prostate and Urological cancers at MSKCC, New York, NY, USA, grants from Swedish Cancer Society grant nr 11-0624 to Lund University, Malmö, Sweden, grants from Fundacion Federico SA grant to Lund University, Malmö, Sweden, grants from FiDiPro grant from TEKES, Finland to IBT, University of Tampere, Tampere, Finland, grants from the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre Program, University of Oxford, Oxford, UK, during the conduct of the study; other from Arctic Partners, outside the submitted work; In addition, Dr. Lilja has a patent free PSA, intact PSA and hK2 assays with royalties paid to OPKO.Dr. Lilja reports grants from National Cancer Institute (R01CA160816; P50-CA92629) to MSKCC, New York, NY, USA, grants from David H. Koch trough the Prostate Cancer Foundation to the The Sidney Kimmel Center for Prostate and Urological cancers at MSKCC, New York, NY, USA, grants from Swedish Cancer Society (nr 11-0624) to Lund University, Malmö, Sweden, grants from Fundacion Federico SA grant to Lund University, Malmö, Sweden, grants from FiDiPro grant from TEKES, Finland to IBT, University of Tampere, Tampere, Finland , grants from the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre Program, University of Oxford, Oxford, UK, during the conduct of the study; In addition, Dr. Lilja has a patent free PSA, intact PSA and hK2 assays with royalties paid to Arctic Partners, and a patent application for a statistical method to detect prostate cancer licensed to Arctic Partners. Dr. Moss reports grants from Rotterdam Prostate Cancer Research Foundation (SWOP) during the conduct of the study. Dr. TAMMELA reports personal fees from Astellas, personal fees from Janssen, personal fees from Orion Pharma, personal fees from AMGEN, outside the submitted work. Dr. Stenman has a patent Determination of free PSA with royalties paid to Perkin-Elmar Wallac. All other authors declare that they have no conflicts of interest.
Figures
Comment in
-
Prostate cancer screening comes of age.Lancet. 2014 Dec 6;384(9959):2004-6. doi: 10.1016/S0140-6736(14)61008-4. Epub 2014 Aug 6. Lancet. 2014. PMID: 25108887 No abstract available.
-
Appraising the European randomized study of screening for prostate cancer: what do the results mean?Asian J Androl. 2015 Mar-Apr;17(2):221-2. doi: 10.4103/1008-682X.142131. Asian J Androl. 2015. PMID: 25432497 Free PMC article.
-
Words of wisdom. Re: Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up.Eur Urol. 2015 Jan;67(1):175. doi: 10.1016/j.eururo.2014.09.048. Epub 2014 Nov 25. Eur Urol. 2015. PMID: 25528396 No abstract available.
-
Words of wisdom. Re: Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up.Eur Urol. 2014 Dec;66(6):1187-8. doi: 10.1016/j.eururo.2014.08.044. Eur Urol. 2014. PMID: 25587592 No abstract available.
-
Although the evidence is not clear, decreases in prostate cancer mortality in specific subgroups of men may be due to screening.Evid Based Med. 2015 Jun;20(3):102. doi: 10.1136/ebmed-2015-110166. Epub 2015 Mar 13. Evid Based Med. 2015. PMID: 25770150 No abstract available.
-
[Early detection of prostate cancer--recommendations after 13 years of follow-up in the European randomised study].Ned Tijdschr Geneeskd. 2015;159:A8677. Ned Tijdschr Geneeskd. 2015. PMID: 25850455 Dutch.
-
Prostate cancer screening in Europe.Lancet. 2015 Apr 18;385(9977):1506. doi: 10.1016/S0140-6736(15)60746-2. Lancet. 2015. PMID: 25933274 No abstract available.
-
Prostate cancer screening in Europe.Lancet. 2015 Apr 18;385(9977):1506. doi: 10.1016/S0140-6736(15)60747-4. Lancet. 2015. PMID: 25933275 No abstract available.
-
Prostate cancer screening in Europe - Authors' reply.Lancet. 2015 Apr 18;385(9977):1507-8. doi: 10.1016/S0140-6736(15)60749-8. Lancet. 2015. PMID: 25933276 No abstract available.
-
Prostate cancer screening in Europe.Lancet. 2015 Apr 18;385(9977):1507. doi: 10.1016/S0140-6736(15)60748-6. Lancet. 2015. PMID: 25933277 No abstract available.
-
Re: Screening and Prostate Cancer Mortality: Results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 Years of Follow-up.J Urol. 2015 Aug;194(2):392. doi: 10.1016/j.juro.2015.05.064. Epub 2015 May 18. J Urol. 2015. PMID: 26195362 No abstract available.
-
Interpreting the Statistics on Potential Benefits of Prostate Cancer Screening.Am Fam Physician. 2017 Apr 1;95(7):412-413. Am Fam Physician. 2017. PMID: 28409604 No abstract available.
References
-
- Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–1328. - PubMed
-
- Draisma G, Boer R, Otto SJ, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003;95(12):868–878. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous