Cocaine exposure reorganizes cell type- and input-specific connectivity in the nucleus accumbens
- PMID: 25108911
- PMCID: PMC4146520
- DOI: 10.1038/nn.3783
Cocaine exposure reorganizes cell type- and input-specific connectivity in the nucleus accumbens
Abstract
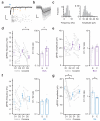
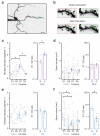
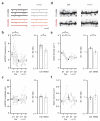
Repeated exposure to cocaine alters the structural and functional properties of medium spiny neurons (MSNs) in the nucleus accumbens (NAc). These changes suggest a rewiring of the NAc circuit, with an enhancement of excitatory synaptic connections onto MSNs. However, it is unknown how drug exposure alters the balance of long-range afferents onto different cell types in the NAc. Here we used whole-cell recordings, two-photon microscopy, optogenetics and pharmacogenetics to show how repeated cocaine exposure alters connectivity in the mouse NAc medial shell. Cocaine selectively enhanced amygdala innervation of MSNs expressing D1 dopamine receptors (D1-MSNs) relative to D2-MSNs. We also found that amygdala activity was required for cocaine-induced changes to behavior and connectivity. Finally, we established how heightened amygdala innervation can explain the structural and functional changes evoked by cocaine. Our findings reveal how exposure to drugs of abuse fundamentally reorganizes cell type- and input-specific connectivity in the NAc.
Figures




References
-
- Humphries MD, Prescott TJ. The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Progress in Neurobiology. 2010;90:385–417. - PubMed
-
- Wilson CJ. In: The Synaptic Organization of the Brain. Shepherd GM, editor. Oxford University Press; 2004.
-
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annual Review of Neuroscience. 2006;29:565–598. - PubMed
-
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nature Reviews. Neuroscience. 2009;10:561–572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

