Morphology and fibre-type distribution in the tongue of the Pogona vitticeps lizard (Iguania, Agamidae)
- PMID: 25109482
- PMCID: PMC4174021
- DOI: 10.1111/joa.12224
Morphology and fibre-type distribution in the tongue of the Pogona vitticeps lizard (Iguania, Agamidae)
Abstract
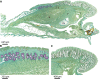
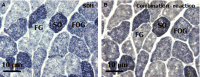
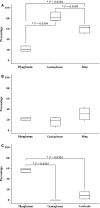
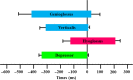
Agamid lizards use tongue prehension for capturing all types of prey. The purpose of this study was to investigate the functional relationship between tongue structure, both surface and musculature, and function during prey capture in Pogona vitticeps. The lack of a detailed description of the distribution of fibre-types in the tongue muscles in some iguanian lizards has hindered the understanding of the functional morphology of the lizard tongue. Three methodological approaches were used to fill this gap. First, morphological analyses were performed (i) on the tongue surface through scanning electron microscopy, and (ii) on the lingual muscle by histological coloration and histochemistry to identify fibre-typing. Secondly, kinematics of prey capture was quantified by using high-speed video recordings to determine the movement capabilities of the tongue. Finally, electromyography (EMG) was used to identify the motor pattern tongue muscles during prey capture. Morphological and functional data were combined to discuss the functional morphology of the tongue in agamid lizards, in relation to their diet. During tongue protraction, M. genioglossus contracts 420 ± 96 ms before tongue-prey contact. Subsequently, Mm. verticalis and hyoglossus contract throughout tongue protraction and retraction. Significant differences are found between the timing of activity of the protractor muscles between omnivorous agamids (Pogona sp., this study) and insectivorous species (Agama sp.), despite similar tongue and jaw kinematics. The data confirm that specialisation toward a diet which includes more vegetal materials is associated with significant changes in tongue morphology and function. Histoenzymology demonstrates that protractor and retractor muscles differ in fibre composition. The proportion of fast glycolytic fibres is significantly higher in the M. hyoglossus (retractor muscle) than in the M. genioglossus (protractor muscle), and this difference is proposed to be associated with differences in the velocity of tongue protrusion and retraction (5 ± 5 and 40 ± 13 cm s(-1) , respectively), similar to Chamaeleonidae. This study provides a way to compare fibre-types and composition in all iguanian and scleroglossan lizards that use tongue prehension to catch prey.
Keywords: Agamidae; capture; electromyography; fibre typing; muscle; tongue.
© 2014 Anatomical Society.
Figures




References
-
- Beisser C, Lemell P, Weisgram J. The dorsal lingual epithelium of Rhinoclemmys pulcherrima incisa (Chelonia, Cryptodira) Anat Rec A Discov Mol Cell Evol Biol. 2004;277:227–235. - PubMed
-
- Bels VL. Evaluating the complexity of the trophic system in Reptilia. In: Bels VL, Gasc J-P, Casinos A, editors. Vertebrate Biomechanics and Evolution. Vol. 12. Oxford: BIOS Scientific Publishers Ltd; 2003. –202.pp. 185
-
- Bels VL, Chardon M, Kardong KV. Biomechanics of the Hyolingual System in Squamata. In: Bels VL, Chardon M, Vandewalle P, editors. Biomechanics of Feeding in Vertebrates. Berlin: Springer; 1994. pp. 197–240. Advances in Comparative and Environmental Physiology.
-
- Bonine KE, Gleeson TT, Garland T. Comparative analysis of fiber-type composition in the iliofibularis muscle of phrynosomatid lizards (Squamata) J Morphol. 2001;250:265–280. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

