The extracellular matrix contributes to mechanotransduction in uterine fibroids
- PMID: 25110476
- PMCID: PMC4106177
- DOI: 10.1155/2014/783289
The extracellular matrix contributes to mechanotransduction in uterine fibroids
Abstract
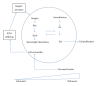
The role of the extracellular matrix (ECM) and mechanotransduction as an important signaling factor in the human uterus is just beginning to be appreciated. The ECM is not only the substance that surrounds cells, but ECM stiffness will either compress cells or stretch them resulting in signals converted into chemical changes within the cell, depending on the amount of collagen, cross-linking, and hydration, as well as other ECM components. In this review we present evidence that the stiffness of fibroid tissue has a direct effect on the growth of the tumor through the induction of fibrosis. Fibrosis has two characteristics: (1) resistance to apoptosis leading to the persistence of cells and (2) secretion of collagen and other components of the ECM such a proteoglycans by those cells leading to abundant disposition of highly cross-linked, disoriented, and often widely dispersed collagen fibrils. Fibrosis affects cell growth by mechanotransduction, the dynamic signaling system whereby mechanical forces initiate chemical signaling in cells. Data indicate that the structurally disordered and abnormally formed ECM of uterine fibroids contributes to fibroid formation and growth. An appreciation of the critical role of ECM stiffness to fibroid growth may lead to new strategies for treatment of this common disease.
Figures


References
-
- Jayes FL, Ma X, Flannery EM, Moutos FT, Guilak F, Leppert PC. Biomechanical Evaluation of Human Uterine Fibroids after Exposure to Purified Clostridial Collagenase. Montreal, Canada: Society for the Study of Reproduction; 2013.
-
- Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260(5111):1124–1127. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

