Anti-inflammatory effects of Lactococcus lactis NCDO 2118 during the remission period of chemically induced colitis
- PMID: 25110521
- PMCID: PMC4126083
- DOI: 10.1186/1757-4749-6-33
Anti-inflammatory effects of Lactococcus lactis NCDO 2118 during the remission period of chemically induced colitis
Abstract
Background: Many probiotic bacteria have been described as promising tools for the treatment and prevention of inflammatory bowel diseases (IBDs). Most of these bacteria are lactic acid bacteria, which are part of the healthy human microbiota. However, little is known about the effects of transient bacteria present in normal diets, including Lactococcus lactis.
Methods: In the present study, we analysed the immunomodulatory effects of three L. lactis strains in vitro using intestinal epithelial cells. L. lactis NCDO 2118 was administered for 4 days to C57BL/6 mice during the remission period of colitis induced by dextran sodium sulphate (DSS).
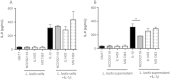
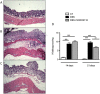
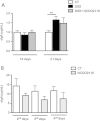
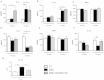
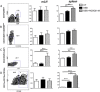
Results: Only one strain, L. lactis NCDO 2118, was able to reduce IL-1β-induced IL-8 secretion in Caco-2 cells, suggesting a potential anti-inflammatory effect. Oral treatment using L. lactis NCDO 2118 resulted in a milder form of recurrent colitis than that observed in control diseased mice. This protective effect was not attributable to changes in secretory IgA (sIgA); however, NCDO 2118 administration was associated with an early increase in IL-6 production and sustained IL-10 production in colonic tissue. Mice fed L. lactis NCDO 2118 had an increased number of regulatory CD4(+) T cells (Tregs) bearing surface TGF-β in its latent form (Latency-associated peptide-LAP) in the mesenteric lymph nodes and spleen.
Conclusions: Here, we identified a new probiotic strain with a potential role in the treatment of IBD, and we elucidated some of the mechanisms underlying its anti-inflammatory effect.
Keywords: Colitis; Cytokines; Lactococcus lactis; Probiotics; Regulatory T cells.
Figures

Similar articles
-
SlpB Protein Enhances the Probiotic Potential of L. lactis NCDO 2118 in Colitis Mice Model.Front Pharmacol. 2021 Dec 20;12:755825. doi: 10.3389/fphar.2021.755825. eCollection 2021. Front Pharmacol. 2021. PMID: 34987390 Free PMC article.
-
Therapeutic Effects of Probiotic Minas Frescal Cheese on the Attenuation of Ulcerative Colitis in a Murine Model.Front Microbiol. 2021 Mar 2;12:623920. doi: 10.3389/fmicb.2021.623920. eCollection 2021. Front Microbiol. 2021. PMID: 33737918 Free PMC article.
-
Immunomodulatory effects of different strains of Lactococcus lactis in DSS-induced colitis.Braz J Microbiol. 2023 Jun;54(2):1203-1215. doi: 10.1007/s42770-023-00928-0. Epub 2023 Feb 23. Braz J Microbiol. 2023. PMID: 36821043 Free PMC article.
-
Protective effects of a novel probiotic strain, Lactococcus lactis ML2018, in colitis: in vivo and in vitro evidence.Food Funct. 2019 Feb 20;10(2):1132-1145. doi: 10.1039/c8fo02301h. Food Funct. 2019. PMID: 30724927
-
Effects of Lactococcus lactis on colorectal cancer in various terms: a narrative review.Ann Med Surg (Lond). 2024 Apr 4;86(6):3503-3507. doi: 10.1097/MS9.0000000000002030. eCollection 2024 Jun. Ann Med Surg (Lond). 2024. PMID: 38846866 Free PMC article. Review.
Cited by
-
Comparative proteomic analysis of four biotechnological strains Lactococcus lactis through label-free quantitative proteomics.Microb Biotechnol. 2019 Mar;12(2):265-274. doi: 10.1111/1751-7915.13305. Epub 2018 Oct 19. Microb Biotechnol. 2019. PMID: 30341804 Free PMC article.
-
The important role of polysaccharides from a traditional Chinese medicine-Lung Cleansing and Detoxifying Decoction against the COVID-19 pandemic.Carbohydr Polym. 2020 Jul 15;240:116346. doi: 10.1016/j.carbpol.2020.116346. Epub 2020 Apr 22. Carbohydr Polym. 2020. PMID: 32475597 Free PMC article. Review.
-
Daikenchuto (TU-100) shapes gut microbiota architecture and increases the production of ginsenoside metabolite compound K.Pharmacol Res Perspect. 2016 Feb 10;4(1):e00215. doi: 10.1002/prp2.215. eCollection 2016 Feb. Pharmacol Res Perspect. 2016. PMID: 26977303 Free PMC article.
-
Surface Proteins of Lactococcus lactis: Bacterial Resources for Muco-adhesion in the Gastrointestinal Tract.Front Microbiol. 2017 Nov 23;8:2247. doi: 10.3389/fmicb.2017.02247. eCollection 2017. Front Microbiol. 2017. PMID: 29218032 Free PMC article. Review.
-
Intake of Lactobacillus delbrueckii (pExu:hsp65) Prevents the Inflammation and the Disorganization of the Intestinal Mucosa in a Mouse Model of Mucositis.Microorganisms. 2021 Jan 5;9(1):107. doi: 10.3390/microorganisms9010107. Microorganisms. 2021. PMID: 33466324 Free PMC article.
References
-
- LeBlanc JG, Aubry C, Cortes-Perez NG, de Moreno de LeBlanc A, Vergnolle N, Langella P, Azevedo V, Chatel J-M, Miyoshi A, Bermúdez-Humarán LG. Mucosal targeting of therapeutic molecules using genetically modified lactic acid bacteria: an update. FEMS Microbiol Lett. 2013;344(1):1–9. - PubMed
-
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. - PubMed
-
- Nielsen OH, Munck LK. Drug insight: aminosalicylates for the treatment of IBD. Nat Clin Pract Gastroenterol Hepatol. 2007;4:160–170. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

