Triheptanoin for glucose transporter type I deficiency (G1D): modulation of human ictogenesis, cerebral metabolic rate, and cognitive indices by a food supplement
- PMID: 25110966
- PMCID: PMC4376124
- DOI: 10.1001/jamaneurol.2014.1584
Triheptanoin for glucose transporter type I deficiency (G1D): modulation of human ictogenesis, cerebral metabolic rate, and cognitive indices by a food supplement
Abstract
Importance: Disorders of brain metabolism are multiform in their mechanisms and manifestations, many of which remain insufficiently understood and are thus similarly treated. Glucose transporter type I deficiency (G1D) is commonly associated with seizures and with electrographic spike-waves. The G1D syndrome has long been attributed to energy (ie, adenosine triphosphate synthetic) failure such as that consequent to tricarboxylic acid (TCA) cycle intermediate depletion. Indeed, glucose and other substrates generate TCAs via anaplerosis. However, TCAs are preserved in murine G1D, rendering energy-failure inferences premature and suggesting a different hypothesis, also grounded on our work, that consumption of alternate TCA precursors is stimulated and may be detrimental. Second, common ketogenic diets lead to a therapeutically counterintuitive reduction in blood glucose available to the G1D brain and prove ineffective in one-third of patients.
Objective: To identify the most helpful outcomes for treatment evaluation and to uphold (rather than diminish) blood glucose concentration and stimulate the TCA cycle, including anaplerosis, in G1D using the medium-chain, food-grade triglyceride triheptanoin.
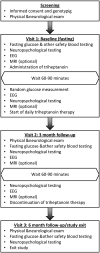
Design, setting, and participants: Unsponsored, open-label cases series conducted in an academic setting. Fourteen children and adults with G1D who were not receiving a ketogenic diet were selected on a first-come, first-enrolled basis.
Intervention: Supplementation of the regular diet with food-grade triheptanoin.
Main outcomes and measures: First, we show that, regardless of electroencephalographic spike-waves, most seizures are rarely visible, such that perceptions by patients or others are inadequate for treatment evaluation. Thus, we used quantitative electroencephalographic, neuropsychological, blood analytical, and magnetic resonance imaging cerebral metabolic rate measurements.
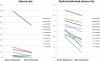
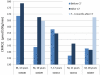
Results: One participant (7%) did not manifest spike-waves; however, spike-waves promptly decreased by 70% (P = .001) in the other participants after consumption of triheptanoin. In addition, the neuropsychological performance and cerebral metabolic rate increased in most patients. Eleven patients (78%) had no adverse effects after prolonged use of triheptanoin. Three patients (21%) experienced gastrointestinal symptoms, and 1 (7%) discontinued the use of triheptanoin.
Conclusions and relevance: Triheptanoin can favorably influence cardinal aspects of neural function in G1D. In addition, our outcome measures constitute an important framework for the evaluation of therapies for encephalopathies associated with impaired intermediary metabolism.
Figures



References
-
- Pascual JM, Wang D, Lecumberri B, et al. GLUT1 deficiency and other glucose transporter diseases. Eur J Endocrinol. 2004 May;150(5):627–633. - PubMed
-
- Marin-Valencia I, Roe CR, Pascual JM. Pyruvate carboxylase deficiency: mechanisms, mimics and anaplerosis. Mol Genet Metab. 2010 Sep;101(1):9–17. - PubMed
-
- Pascual JM, Van Heertum RL, Wang D, Engelstad K, De Vivo DC. Imaging the metabolic footprint of Glut1 deficiency on the brain. Ann Neurol. 2002 Oct;52(4):458–464. - PubMed
-
- Pascual JM, Campistol J, Gil-Nagel A. Epilepsy in inherited metabolic disorders. Neurologist. 2008 Nov;14(6 Suppl 1):S2–S14. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- UL1 TR001105/TR/NCATS NIH HHS/United States
- RR024982/RR/NCRR NIH HHS/United States
- MH084021/MH/NIMH NIH HHS/United States
- NS065640/NS/NINDS NIH HHS/United States
- NS078059/NS/NINDS NIH HHS/United States
- UL1 RR024982/RR/NCRR NIH HHS/United States
- F32 NS065640/NS/NINDS NIH HHS/United States
- NS067015/NS/NINDS NIH HHS/United States
- NS077015/NS/NINDS NIH HHS/United States
- P41 RR002584/RR/NCRR NIH HHS/United States
- R01 NS067015/NS/NINDS NIH HHS/United States
- R01 NS077015/NS/NINDS NIH HHS/United States
- RR002584/RR/NCRR NIH HHS/United States
- U54 NS078059/NS/NINDS NIH HHS/United States
- R01 MH084021/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

