Immunophenotyping and efficacy of low dose ATG in non-sensitized kidney recipients undergoing early steroid withdrawal: a randomized pilot study
- PMID: 25111080
- PMCID: PMC4128673
- DOI: 10.1371/journal.pone.0104408
Immunophenotyping and efficacy of low dose ATG in non-sensitized kidney recipients undergoing early steroid withdrawal: a randomized pilot study
Abstract
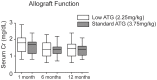
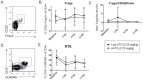
Rabbit antithymocyte globulin (ATG) is commonly used as an induction therapy in renal transplant recipients, but the ideal dosage in tacrolimus-based early steroid withdrawal protocols has not been established. The purpose of this pilot study was to determine the immunophenotyping and efficacy of lower dose ATG in low immunological-risk kidney transplant recipients. In this prospective study, 45 patients were randomized (1∶1) to our standard dose ATG (total dose 3.75 mg/kg)(sATG) vs. lower dose 2.25 mg/kg (lowATG). All patients underwent early steroid withdrawal within 7 days. The primary end point was biopsy-proven acute rejection at 12 months. Prospective immunophenotyping of freshly isolated PBMCs was performed at baseline, 3, 6, 12 months post-transplant. The rate of acute rejection was 17% and 10% in the sATG and lowATG, respectively. Effector memory T cells, Tregs and recent thymic emigrants T cells had similar kinetics post-transplant in both groups. No statistically significant differences were found in graft survival, patient survival or infections between the two groups, though there was a non-significant increase in leukopenia (43%v s. 30%), CMV (8% vs. 0) and BK (4% vs. 0) infections in sATG group vs. lowATG. In sum, in low immunological risk kidney recipients undergoing steroid withdrawal, low dose ATG seems to be efficacious in preventing acute rejection and depleting T cells with potentially lower infectious complications. A larger study is warranted to confirm these findings.
Trial registration: ClinicalTrials.gov NCT00548405.
Conflict of interest statement
Figures


References
-
- Halloran PF (2004) Immunosuppressive drugs for kidney transplantation. N Engl J Med 351: 2715–2729. - PubMed
-
- Matas AJ, Smith JM, Skeans MA, Lamb KE, Gustafson SK, et al. (2013) OPTN/SRTR 2011 Annual Data Report: kidney. Am J Transplant 13 Suppl 1: 11–46. - PubMed
-
- Meier-Kriesche HU, Li S, Gruessner RW, Fung JJ, Bustami RT, et al. (2006) Immunosuppression: evolution in practice and trends, 1994–2004. Am J Transplant 6: 1111–1131. - PubMed
-
- Kirk AD (2006) Induction immunosuppression. Transplantation 82: 593–602. - PubMed
-
- Thiyagarajan U, Ponnuswamy A, Bagul A (2013) Thymoglobulin and its use in renal transplantation: a review. American journal of nephrology 37: 586–601. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

