18F-glutathione conjugate as a PET tracer for imaging tumors that overexpress L-PGDS enzyme
- PMID: 25111383
- PMCID: PMC4128654
- DOI: 10.1371/journal.pone.0104118
18F-glutathione conjugate as a PET tracer for imaging tumors that overexpress L-PGDS enzyme
Abstract
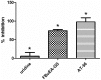
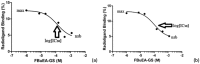
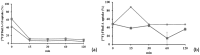
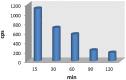
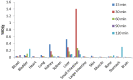
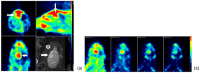
Lipocalin-type prostaglandin D synthase (L-PGDS) has been correlated with the progression of neurological disorders. The present study aimed at evaluating the imaging potency of a glutathione conjugate of fluorine-18-labeled fluorobutyl ethacrynic amide ([18F]FBuEA-GS) for brain tumors. Preparation of [18F]FBuEA-GS has been modified from the -4-tosylate derivative via radiofluorination in 5% radiochemical yield. The mixture of nonradioactive FBuEA-GS derived from a parallel preparation has be resolved to two isomers in a ratio of 9:1 using analytic chiral reversed phase high performance liquid chromatography (RP-HPLC). The two fluorine-18-labeled isomers purified through nonchiral semipreparative RP-HPLC as a mixture were studied by assessing the binding affinity toward L-PGDS through a gel filtration HPLC, by analyzing radiotracer accumulation in C6 glioma cells, and by evaluating the imaging of radiotracer in a C6 glioma rat with positron emission tomography. The inhibition percentage of the production of PGD2 from PGH2 at the presence of 200 µM of FBuEA-GS and 4-Dibenzo[a,d]cyclohepten-5-ylidene-1-[4-(2H-tetrazol-5-yl)butyl]piperidine (AT-56) were 74.1 ± 4.8% and 97.6 ± 16.0%, respectively. [18F]FBuEA-GS bound L-PGDS (16.3-21.7%) but not the isoform, microsomal prostaglandin E synthase 1. No binding to GST-alpha and GST-pi was observed. The binding strength between [18F]FBuEA-GS and L-PGDS has been evaluated using analytic gel filtration HPLC at the presence of various concentrations of the cold competitor FBuEA-GS. The contrasted images indicated that the radiotracer accumulation in tumor lesions is probably related to the overexpression of L-PGDS.
Conflict of interest statement
Figures


Similar articles
-
Investigation of brain tumors using (18)F-fluorobutyl ethacrynic amide and its metabolite with positron emission tomography.Onco Targets Ther. 2015 Jul 24;8:1877-85. doi: 10.2147/OTT.S78404. eCollection 2015. Onco Targets Ther. 2015. PMID: 26244025 Free PMC article.
-
Synthesis and evaluation of [18F]Fluorobutyl ethacrynic amide: a potential PET tracer for studying glutathione transferase.Bioorg Med Chem Lett. 2012 Jun 15;22(12):3998-4003. doi: 10.1016/j.bmcl.2012.04.091. Epub 2012 Apr 30. Bioorg Med Chem Lett. 2012. PMID: 22607679
-
Biochemical, functional, and pharmacological characterization of AT-56, an orally active and selective inhibitor of lipocalin-type prostaglandin D synthase.J Biol Chem. 2009 Mar 20;284(12):7623-30. doi: 10.1074/jbc.M808593200. Epub 2009 Jan 8. J Biol Chem. 2009. PMID: 19131342 Free PMC article.
-
Lipocalin-Type Prostaglandin D2 Synthase Protein- A Central Player in Metabolism.Pharm Res. 2022 Nov;39(11):2951-2963. doi: 10.1007/s11095-022-03329-4. Epub 2022 Jul 8. Pharm Res. 2022. PMID: 35799081 Review.
-
Lipocalin-type and hematopoietic prostaglandin D synthases as a novel example of functional convergence.Prostaglandins Other Lipid Mediat. 2002 Aug;68-69:375-82. doi: 10.1016/s0090-6980(02)00042-4. Prostaglandins Other Lipid Mediat. 2002. PMID: 12432930 Review.
Cited by
-
Investigation of brain tumors using (18)F-fluorobutyl ethacrynic amide and its metabolite with positron emission tomography.Onco Targets Ther. 2015 Jul 24;8:1877-85. doi: 10.2147/OTT.S78404. eCollection 2015. Onco Targets Ther. 2015. PMID: 26244025 Free PMC article.
-
A perspective on the radiopharmaceutical requirements for imaging and therapy of glioblastoma.Theranostics. 2021 Jul 6;11(16):7911-7947. doi: 10.7150/thno.56639. eCollection 2021. Theranostics. 2021. PMID: 34335972 Free PMC article. Review.
-
PET Molecular Imaging in Drug Development: The Imaging and Chemistry Perspective.Front Med (Lausanne). 2022 Feb 28;9:812270. doi: 10.3389/fmed.2022.812270. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35295604 Free PMC article. Review.
-
Synthesis and evaluation of ortho-[18F] fluorocelecoxib for COX-2 cholangiocarcinoma imaging.Drug Des Devel Ther. 2018 May 24;12:1467-1478. doi: 10.2147/DDDT.S161718. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 29872269 Free PMC article.
-
Synthesis and Biological Evaluation of an (18)Fluorine-Labeled COX Inhibitor--[(18)F]Fluorooctyl Fenbufen Amide--For Imaging of Brain Tumors.Molecules. 2016 Mar 21;21(3):387. doi: 10.3390/molecules21030387. Molecules. 2016. PMID: 27007363 Free PMC article.
References
-
- McCarthy BJ, Kruchko C, Dolecek TA (2013) Theimpact of the benign cancer registries amendment act (public law 107–260) on non-malignant brain and central nervous system tumor incidence trends. J Registry Manag 40 (1) 32–35. - PubMed
-
- Corroyer-Dulmont A, Pérès EA, Petit E, Guillamo JS, Varoqueaux N, et al. (2013) Detection of glioblastoma response to temozolomide combined with bevacizumab based on μMRI and μPET imaging reveals [18F]-fluoro-L-thymidine as an early and robust predictive marker for treatment efficacy. Neuro Oncol 15: 41–56. - PMC - PubMed
-
- Chen W, Delaloye S, Silverman DH, Geist C, Czernin J, et al. (2007) Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: a pilot study. J Clin Oncol 25: 4714–2721. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

