Nonmyeloablative allogeneic hematopoietic stem cell transplantation for GATA2 deficiency
- PMID: 25111582
- PMCID: PMC4253545
- DOI: 10.1016/j.bbmt.2014.08.004
Nonmyeloablative allogeneic hematopoietic stem cell transplantation for GATA2 deficiency
Abstract
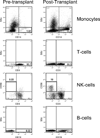
We treated 14 patients with GATA2 deficiency using a nonmyeloablative allogeneic hematopoietic stem cell transplantation regimen. Four patients received peripheral blood stem cells from matched related donors (MRD), 4 patients received peripheral blood stem cells from matched unrelated donors (URD), 4 patients received hematopoietic stem cells from umbilical cord blood donors (UCB), and 2 patients received bone marrow cells from haploidentical related donors. MRD and URD recipients received conditioning with 3 days of fludarabine and 200 cGy total body irradiation (TBI). Haploidentical related donor recipients and UCB recipients received cyclophosphamide and 2 additional days of fludarabine along with 200 cGY TBI. MRD, URD, and UCB recipients received tacrolimus and sirolimus for post-transplantation immunosuppression, whereas haploidentical recipients received high-dose cyclophosphamide followed by tacrolimus and mycophenolate mofetil. Eight patients are alive with reconstitution of the severely deficient monocyte, B cell, and natural killer cell populations and reversal of the clinical phenotype at a median follow-up of 3.5 years. Two patients (1 URD recipient and 1 UCB recipient) rejected the donor graft and 1 MRD recipient relapsed with myelodysplastic syndrome after transplantation. We are currently using a high-dose conditioning regimen with busulfan and fludarabine in patients with GATA2 deficiency to achieve more consistent engraftment and eradication of the malignant myeloid clones.
Keywords: Familial acute myelogenous leukemia; GATA2; Hematopoietic stem cell transplantation; Myelodysplastic syndrome.
Published by Elsevier Inc.
Conflict of interest statement
Conflict of Interest: The authors declare no conflict of interest.
Figures



References
-
- Vicente C, Vazquez I, Conchillo A, Garcia-Sanchez MA, Marcotegui N, Fuster O, et al. Overexpression of GATA2 predicts an adverse prognosis for patients with acute myeloid leukemia and it is associated with distinct molecular abnormalities. Leukemia. 2012;26:550–554. - PubMed
-
- Ostergaard P, Simpson MA, Connell FC, Steward CG, Brice G, Woollard WJ, et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome) Nat Genet. 2011;43:929–931. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

